Abstract
Sunscreens have been shown to protect against UVR-induced DNA damage in human skin under laboratory conditions. We presently extended these observations to real-life conditions in volunteers after their ordinary exposure habits during summer holidays. Volunteers were randomly assigned to a control group and an educated group supplied with a SPF ≥50 sunscreen and receiving instructions for use. A questionnaire was used to determine the extent of exposure. No difference in average solar UVR exposure was found between the two groups. DNA photoprotection was first assessed by, to our knowledge, a previously unreported noninvasive assay on the basis of the quantification of pyrimidine dimers released by DNA repair in urine. Damage was also quantified in the nuclear DNA extracted from the roof of suction blisters collected after recreational exposure. The urinary concentration of photoproducts was significantly higher in the control than in the educated group. The same trend was observed for the level of photoproducts in the DNA from suction blisters. The unambiguous observation of an efficient photoprotection against DNA damage afforded by sunscreen under real-life conditions provides strong support for the efficiency of the sunscreens. In addition, the results validate the use of urinary DNA photoproducts as a noninvasive assay applicable to photoprotection.
Introduction
Overexposure to sunlight is unambiguously associated with the onset of skin cancer (El Ghissassi et al., 2009; Narayanan et al., 2010). This role of solar radiation and in particular of its UVR portion is mostly explained by the induction of DNA damage such as pyrimidine dimers (Cadet and Douki, 2018; Kciuk et al., 2020). These DNA photoproducts participate in the initiation of the tumoral process as shown by the specific mutational signature of basal (Bonilla et al., 2016) and squamous (Durinck et al., 2011) carcinomas and melanoma (Hayward et al., 2017; Hodis et al., 2012). Preventing the formation of this DNA damage is an attractive photoprotection strategy against skin cancer. Sunscreens, in addition to seeking shade and wearing clothes, are commonly used to avoid the deleterious effects of solar UVR. The assessment of the protection afforded by solar products is primarily measured against erythema (International Standardization Organization, 2019). In addition, some studies have shown that sunscreens also afford unambiguous protection against DNA damage (Olsen et al., 2017). These studies are mostly laboratory works where volunteers are exposed to well-defined acute doses of simulated solar UVR, often over a short period. In this study, we aimed to assess the protection of DNA afforded by a novel phenylene bis-diphenyltriazine–based FPF50+ photoprotector (Bacqueville et al., 2022, 2021) in real-life conditions, namely under ordinary exposure habits during summer vacation.
Two complementary assays were used to estimate the extent of DNA damage, both on the basis of sensitive and specific high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) measurements (Douki, 2013). The first was the detection of pyrimidine dimers in nuclear DNA. This was performed on the roof of suction blisters, an approach that is less invasive for the skin and the donor than classical biopsies (Josse et al., 2020, 2018). The second and innovative method was the quantification of the pyrimidine dimers released in urine by the DNA-repair machinery (Reynaud et al., 2022). This study, to our knowledge, shows a previously unreported application of this noninvasive strategy to the assessment of photoprotection. For comparative purposes, we also designed a semiquantitative method, on the basis of a smartphone application record, for the evaluation of the extent of exposure received by each volunteer.
Results
Evaluation of the exposure of volunteers
In this study, we aimed to assess the extent of DNA damage in volunteers during their holidays without interfering with their exposure habits. The selected volunteers exhibited skin phototype III or IV to minimize the impact of this parameter on the results. Urine and suction blisters were collected 1 or 2 days after the end of the exposure period upon an intermediary visit (visit 2). The time between the inclusion visit (visit 1) and visit 2 varied between 7 and 39 days depending on the volunteer, irrespective of the group they were assigned to. Because all samples were collected at visit 2 at the end of exposure, the amount of DNA photoproducts had the same relevance in all volunteers. Because the exposure period varied largely from one volunteer to the other, we felt necessary to evaluate the dose of sunlight. This was made possible by the design of an exposure score on the basis of a questionnaire for each participant of the study (Table 1 and Figure 1). This assessment of exposure is obviously only semiquantitative. However, it was relevant enough to show the absence of difference in sun exposure score between the control group and the educated group (control group: mean ± SD = 22.0 ± 13.9; educated group: mean ± SD = 23.5 ± 21.8). It should also be stressed that individual values of the scores were not used in the subsequent study and the assessment of photoprotection.
Table 1.
Sun Exposition Score for the Two Investigated Groups
| Exposition Score | ||||||
|---|---|---|---|---|---|---|
| Group | n | Mean | SD | Median | Minimum | Maximum |
| Educated Group | 11 | 23.5 | 21.8 | 16.8 | 5.40 | 81.0 |
| Control Group | 12 | 22.0 | 13.9 | 20.6 | 6.00 | 48.0 |
Values were obtained from a questionnaire taking into account the vacation location, the weather, the exposure duration, and the clothing.
Figure 1.
Exposition scores determined in the two investigated groups (control group and educated group). The graph is presented with the median and 25th–75th percentiles range. The difference is not statistically significant.
Assessment of DNA photoprotection by urinary markers
Photoproducts arising from DNA repair could be unambiguously detected in urine after exposure in both control and educated groups. A recently developed method (Reynaud et al., 2022) relies on the isolation and enzymatic hydrolysis of the oligonucleotides released by the DNA-repair machinery. In that respect, it is more quantitative than the previous urinary measurements based on 32P post-labeling (Kotova et al., 2005; Le Curieux and Hemminki, 2001; Petersen et al., 2014) or a recent HPLC-MS/MS method (Lerche et al., 2022). These techniques target only photoproducts released from DNA-repair products by constitutive nucleases the efficiency of which is unknown. It is also expected that because accurate HPLC-MS/MS analyses with isotopic dilution are used, the present technique is more quantitative than immunoassay used in earlier quantification of urinary cyclobutane pyrimidine dimers (CPDs) (Ahmad et al., 1999; Cooke et al., 2001)
Thymine–thymine (TT) CPDs were detected in significant amounts in all urine samples from exposed volunteers in the control group and 7 of 11 from the educated group (Figure 2). In contrast, very small peaks were observed at the retention time of TT 64PP. Because of the weakness of the signal, the ratio between the three monitored fragmentation reactions could not be compared with that of the standard. In addition, those HPLC-MS/MS peaks were observed both before and after exposure. We thus decided not to consider TT 64PP and to focus on TT CPD. Interestingly, the number of samples where TT CPD was below the detection limit was three times larger before than after recreational exposure. The concentration of creatinine was determined in all samples and used as a normalization factor. This widely applied correction procedure allowed us to compensate for differences in volumes of urine produced by each donor. The interest of this approach is shown by the large variability in the creatinine content, with a variation coefficient of 50% between all samples and a ratio of 18 between the largest and the lowest values.
Figure 2.
HPLC-MS/MS detection of TT-CPD in the urine of volunteers after summer recreational exposure. The color code corresponds to the three different fragmentation reactions monitored during the analysis (- m/z 545 → 79, - m/z 545 → 195, - m/z 545 → 447). The vertical axis represents the intensity of the signals expressed in cps. Oligonucleotides produced by DNA repair were isolated and hydrolyzed, and the samples were analyzed by HPLC-MS/MS. The chromatograms correspond to the sample with the lowest (min) and largest (max) concentrations in (a) the control and (b) the educated groups. The corresponding volunteers are number 5, 16, 17, and 21. cps, count per second; HPLC-MS/MS, high-performance liquid chromatography–tandem mass spectrometry; max, maximum; min, minimum; TT CPD, thymine–thymine cyclobutane pyrimidine dimer.
Before exposure, the urinary concentrations of TT CPD were below 1 nM for 75% of the volunteers. This value was exceeded only in six individuals, likely because they did not strictly follow the instruction of avoiding direct exposure to UVR before the beginning of the study. Statistical analysis of the normalized TT CPD concentrations measured after sun exposure (raw data are provided in Tables 2 and 3) showed first that the values were larger after than before recreational exposure for the control group (P < 0.001). This difference was not statistically significant in the educated group. In addition, no difference was observed for the two groups before exposure. When the two exposed groups were compared, the TT CPD urinary concentration was found to be significantly lower (P = 0.023) in the educated group than in the control group (Figure 3). The increase in TT CPD due to holiday exposure was six times lower for the educated group than for the control group.
Table 2.
Concentration in TT CPD (nM) and Creatinine (mM) in the Urine of Control Volunteers before and after Recreational Exposure
| Volunteer | Creatinine (mM) |
CPD (nM) |
CPD (pmol/μmol Creatinine) |
|||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| 2 | 25 | 11.8 | 0.9 | 2.0 | 0.04 | 0.17 |
| 4 | 11 | 24.9 | 0.8 | 2.8 | 0.08 | 0.11 |
| 5 | 9.2 | 12.1 | n.d. | 1.1 | n.d. | 0.09 |
| 8 | 4.16 | 5.04 | n.d. | 2.6 | n.d. | 0.52 |
| 9 | 6.9 | 5.54 | 0.6 | 1.7 | 0.08 | 0.3 |
| 10 | 13.2 | 12.7 | 4.6 | 10.4 | 0.35 | 0.82 |
| 15 | 2.59 | 3.3 | n.d. | 5.1 | n.d. | 1.54 |
| 16 | 16 | 12.9 | n.d. | 26.7 | n.d. | 2.07 |
| 19 | 5.48 | 8.8 | n.d. | 3.6 | n.d. | 0.41 |
| 20 | 4.16 | 9.21 | n.d. | 4.0 | n.d. | 0.43 |
| 22 | 11 | 9.72 | n.d. | 11.5 | n.d. | 1.18 |
| 23 | 7.92 | 17 | 3.3 | 11.3 | 0.41 | 0.66 |
Abbreviations: CPD, cyclobutene pyrimidine dimer; n.d., not detected; TT CPD, thymine–thymine cyclobutene pyrimidine dimer.
Normalized values are expressed in pmol TT CPD per μmol, μM creatinine, and pmol TT CPD per μmol creatinine.
Table 3.
Concentration in TT CPD (nM) and Creatinine (mM) in the Urine of Educated Volunteers before and after Recreational Exposure
| Volunteer | Creatinine (mM) |
CPD (nM) |
CPD (pmol/μmol Creatinine) |
|||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| 1 | 8.06 | 12.8 | 0.4 | 4.8 | 0.05 | 0.37 |
| 3 | 1.75 | 10.4 | n.d. | n.d. | n.d. | n.d. |
| 6 | 10.2 | 15.3 | 2.9 | n.d. | 0.28 | n.d. |
| 7 | 9.9 | 19.3 | n.d. | n.d. | n.d. | n.d. |
| 11 | 6.08 | 11.1 | 0.6 | 2.5 | 0.10 | 0.23 |
| 12 | 1.36 | 3.48 | n.d. | n.d. | n.d. | n.d. |
| 13 | 9.39 | 16.7 | n.d. | 3.6 | n.d. | 0.22 |
| 14 | 9.83 | 5.14 | 1.7 | 2.1 | 0.17 | 0.41 |
| 17 | 3.61 | 4.06 | 0.0 | 0.7 | n.d. | 0.17 |
| 18 | 12.2 | 12.2 | 4.1 | 2.6 | 0.34 | 0.21 |
| 21 | 13.4 | 12.7 | 2.3 | 7.5 | 0.18 | 0.59 |
Abbreviations: CPD, cyclobutene pyrimidine dimer; n.d., not detected; TT CPD, thymine–thymine cyclobutene pyrimidine dimer.
Normalized values are expressed in pmol TT CPD per μmol, μM creatinine, and pmol TT CPD per μmol creatinine.
Figure 3.
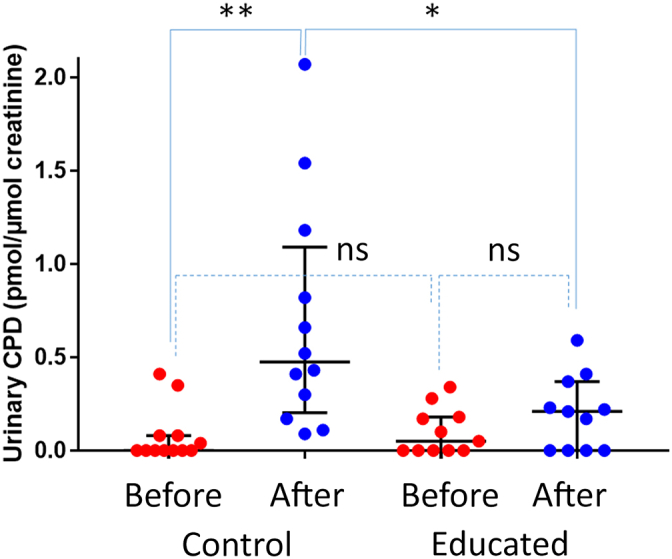
The concentration of TT CPDs in the urine of the control and educated groups before and after recreational exposure to solar UVR. Data are median with 25th–75th percentiles range (n = 12 for control, and n = 11 for educated). A covariance analysis on the change between V1 (before) and V2 (after) with group as a fixed factor and baseline as a covariate was made. Comparisons between LSMeans were realized to perform intragroup and intergroup analyses. ns denotes nonsignificant difference. ∗P < 0.050 and ∗∗P < 0.010. TT CPD, thymine–thymine cyclobutene pyrimidine dimer; V1, visit 1; V2, visit 2.
Assessment of genomic photoprotection in the DNA from suction blisters
The quantification of photoproducts in the DNA extracted from the roof of suction blisters has previously been used to assess the DNA-protection factor of solar products under laboratory conditions (Josse et al., 2020, 2018). The same technique was used in this study with natural UVR. CPDs were present in all the DNA samples extracted from suction blisters collected after exposure (raw data are provided in Table 4). Not only TT CPDs but also the highly mutagenic analog thymine–cytosine CPDs were unambiguously detected and accurately quantified. Interestingly, a statistically significant correlation was found between the amount of the two photoproducts in the DNA of each volunteer (Spearman r = 0.851, P < 0.001). This observation rules out a major role of the analytical procedure in the variance of the results. The latter is more likely due to differences in exposure patterns and skin properties. The absence of 64PP and their Dewar valence isomers could be explained by their fast repair rate. Both CPDs were found to be at lower levels in the DNA from the educated group than in those from the control group (Figure 4). It may be added that a good correlation was found between the levels of TT CPD in nuclear DNA and the urinary concentrations for the protecting group (Spearman r = 0.745, P = 0.012). No such statistical significance was found in the control group, likely because of a few outliers in the urinary concentrations.
Table 4.
Content in TT CPD and TC CPD in the DNA Extracted from the Suction Blisters Collected in Educated and Control Groups after Recreational Exposure
| Educated |
Control |
||||
|---|---|---|---|---|---|
| Volunteer | TT CPD | TC CPD | Volunteer | TT CPD | TC CPD |
| T02 02 | 0.27 | 0.14 | T02 01 | 0.32 | 0.04 |
| T02 04 | 0.16 | 0.08 | T02 03 | 0.19 | 0.13 |
| T02 05 | 0.72 | 0.30 | T02 06 | 0.26 | 0.20 |
| T02 08 | 0.41 | 0.17 | T02 07 | 0.20 | 0.09 |
| T02 09 | 0.94 | 0.40 | T02 11 | 0.30 | 0.12 |
| T02 10 | 0.14 | 0.06 | T02 12 | 0.26 | 0.11 |
| T02 15 | 0.45 | 0.20 | T02 13 | 0.28 | 0.15 |
| T02 16 | 0.51 | 0.21 | T02 14 | 0.35 | 0.16 |
| T02 19 | 0.47 | 0.19 | T02 17 | 0.09 | 0.09 |
| T02 20 | 0.35 | 0.17 | T02 18 | 0.51 | 0.20 |
| T02 22 | 0.51 | 0.30 | T02 21 | 0.37 | 0.17 |
| T02 23 | 0.37 | 0.19 | |||
Abbreviations: CPD, cyclobutene pyrimidine dimer; TC CPD, thymine–cytosine cyclobutene pyrimidine dimer; TT CPD, thymine–thymine cyclobutene pyrimidine dimer.
Data are expressed in number of CPD per million normal bases.
Figure 4.

Level of TT and TC CPDs in the DNA extracted from suction blisters in control and educated groups after recreational exposure to solar UVR. Data are median with 25th–75th percentiles range (n = 12 for control, and n = 11 for educated). ∗P < 0.050 (TT CPD P = 0.047; TC CPD P = 0.047). CPD, cyclobutene pyrimidine dimer; TC, thymine–cytosine; TT, thymine–thymine.
Discussion
The efficiency of sun-protection products against skin cancer is a constant matter of debate (Paul, 2019), in contrast to the prevention of sunburn, which is the basis of the definition of protection factors of sunscreens. Cancer has a long-term effect and cannot be compared with erythema, which takes place within hours. In addition, the biological responses involved in the two phenomena are different. A direct evaluation of the effect of sunscreens in the prevention of skin cancer is thus needed. Whereas a long-term interventional study showed that sunscreens could prevent both squamous cell carcinoma (Green et al., 1999) and melanoma (Green et al., 2011), epidemiological studies have provided contradictory answers (Rueegg et al., 2019; Silva et al., 2018). The correlation between the use of sunscreen and melanoma induction is seen as negative in some studies, pointing to a protective effect, whereas others suggest an increased induction. The latter observation is unexpected but could be explained by the longer exposure time in populations using sunscreen (Autier et al., 2007). Another confounding parameter is the amount of sunscreen applied by users, which is two or three times lower than that in the SPF measurement procedure (Autier et al., 2001; Neale et al., 2002; Petersen et al., 2013).
Monitoring deleterious cellular events can be used as a more direct assessment of the protection afforded by sunscreen against UVR-induced skin carcinogenesis. For example, evidence has been provided for the prevention of immunosuppression by sunscreens (Chen et al., 2016; Moyal and Fourtanier, 2008). In addition, several works have shown the reduced level of DNA photoproducts in the skin biopsies or roof of suction blisters of volunteers exposed to simulated solar UVR under laboratory conditions (Bacqueville et al., 2015; Freeman et al., 1988; Josse et al., 2020, 2018; van Praag et al., 1993; Young et al., 2000, 2018). This approach is efficient but invasive. An assay was recently developed for the quantification of the photoproducts released in biological fluids by the DNA-repair machinery (Reynaud et al., 2022). In this study, it was applied to samples collected from volunteers after a period of summer vacation. For comparative purposes, a more classical quantification of photoproducts in genomic DNA of skin was performed.
Both methods for the evaluation of the extent of DNA damage led to the same unambiguous conclusion of a reduced genotoxic insult in the group properly using the provided sunscreen. The TT CPD urinary concentrations and CPD levels in DNA extracted from suction blisters were found to be larger in the control group than in the educated group after exposure. It may thus be concluded that the novel phenylene bis-diphenyltriazine–based photoprotector (Bacqueville et al., 2022, 2021) used in this study and likely most of the commercial SPF ≥50 products may afford significant protection against DNA damage. Another interesting outcome of this work is the validation of the urinary DNA-repair products as biomarkers in human photobiology. A major argument is the correlation found between the amount of nuclear DNA damage and DNA-repair products released in urine. This noninvasive assay will facilitate the establishment of genoprotective properties of sunscreens in future clinical studies.
Our study shows previously unreported proof in real-life conditions of the DNA-protecting properties of the sunscreen. However, the two major weaknesses of this study are the small size of the studied group and the wide range of exposure times. For this latter reason, it was necessary to check that the two groups had similar span of exposure scores. Yet, the fact that significant differences between the two groups could be observed despite these limitations shows the interest and the strength of the reported strategy. Better control of the exposure and of the applied amount of product, on a larger population, would enable accurate determination of the anticarcinogenic properties of solar products with a noninvasive technique.
Materials and Methods
Recruitment of volunteers and collection of urine and suction blisters
The study was carried out in Toulouse (France) on 23 volunteers—men or women aged 18–55 years—meeting several criteria such as absence of skin disease, no use of photosensitizing drugs, and no known sun-related syndrome, etc. In agreement with the French law (ordonnance n° 2016-800 June 16, 2016 and decree n° 2017-884 May 9, 2017), this study could not be submitted to an ethics committee. The French law considers that studies evaluating cosmetic products do not have to be submitted to an ethical committee. The study was performed in a clinical research center that is accredited by the French National Agency for Safety of Medicinal Products in compliance with the Declaration of Helsinki (1964) and its subsequent revisions and in the spirit of Good Clinical Practice. All volunteers provided written and informed consent. The consent form clearly explained the number and the type of sampling. All samples were anonymized before analysis. Demographic details are provided as supporting information (Table 5). The subjects had to have the habit of using little sunscreen or using low-protection factors (SPF <25) during sun exposure. They also had to plan outdoor solar exposure during their summer holidays. The study was between the end of June 2021 and mid-September 2021, and the latitudes of the volunteers' vacations were between 39.5 and 49.0. They were asked to expose themselves for at least 3 hours a day during the 5 days preceding the end of the study. Finally, they had to agree not to expose themselves to UVR (natural or artificial) before their scheduled beginning of vacation.
Table 5.
Demographic Information on the Volunteers Involved in the Study
| Age | ||||||
|---|---|---|---|---|---|---|
| Group | n | Mean | SD | Median | Minimum | Maximum |
| Educated group | 11 | 37.7 | 8.9 | 38 | 23 | 51 |
| Control group | 12 | 37.9 | 10.3 | 37.5 | 21 | 51 |
| Sex | |||
|---|---|---|---|
| Number (frequency %) | Male | female | Total |
| Educated group | 1 (9.1) | 10 (90.9) | 11 |
| Control group | 1 (8.3) | 11 (91.7) | 12 |
| Total | 2 | 21 | 23 |
| Phototype (Fitzpatrick) | |||
|---|---|---|---|
| Number (frequency %) | III | IV | Total |
| Educated group | 10 (90.9) | 1 (9.1) | 11 |
| Control group | 9 (75) | 3 (25) | 12 |
| Total | 19 | 4 | 23 |
After the selection, the volunteers were randomly included in two groups. The first was a control group in which individuals followed their ordinary exposure and photoprotection habits. The second group, referred to as educated, received SPF ≥50 sunscreen (Intense Protect 50+ Avene) and received instruction on its use. The following three visits were organized during the study:
-
•
Visit 1: Inclusion (day 1), during which a urine sample was collected to determine the basal level of urinary markers;
-
•
Visit 2: Intermediate visit (between 7 and 39 days), corresponding to the end of the exposure. The duration of the latter varied from one volunteer to another, but the visit always took place <2 days after the end of the exposure. During this second visit, the postexposure urine sample was collected. A suction blister was also performed on each person's forearm, and the roof was removed.
-
•
Visit 3: This end-of-study visit allowed the evaluation of healing and is done at visit 2 + 15 days ± 2 days.
Evaluation of the outdoor sun exposure
Most of the volunteers had their summer holidays in areas with intense solar UVR (South of France and Spain). A dedicated smartphone application was designed for the study. Through the application, the subjects each day recorded information relative to the duration of their sun exposure, the weather, and their type of clothing. A global exposure score that takes into account the vacation location, the weather, the exposure duration, and the clothing was computed (each parameter is scaled on five levels). The score was calculated by the formula: S = A × B × C × D, where A is the exposition time (1 for < 1 hour, 2 for 1–3 hours, 3 for 3–5 hours, and 4 for >5 hours), B is the weather state (3, sunny weather; 2, variable weather; and 1, cloudy weather), C is the clothing (0.9, bikini bathing short; 0.5, t-shirt short dress; and 0.15, long clothes), and D is localization (2 for South Europe Italy, Spain…; 1 for France). The final score is the sum of the last five holiday scores.
Quantification of photoproducts
Urinary photoproducts (TT CPDs and TT 64PP) released upon DNA repair were quantified using a recently reported assay (Reynaud et al., 2022). Briefly, triethylammonium acetate was added to 100 μl of urine, together with a known amount of oligonucleotides bearing ethenoadenine used as the internal standard for the overall recovery. The sample was purified on HRX-100 solid phase extraction columns using mixtures of 50 mM triethylammonium acetate in water and methanol as eluent. Isolated oligonucleotides (internal standard and photoproducts-bearing oligonucleotides produced by DNA repair) were enzymatically hydrolyzed. After filtration (0.2 μm), the samples were freeze dried. They were then solubilized in a solution containing isotopically labeled (M+12) photoproducts used as the internal standard for the mass spectrometry calibration curve.
Suction blisters (6 mm diameter) were induced on the forearm of the volunteers by applying negative pressure to the skin (300 mbar) for approximately 3 hours. After the blisters were formed, the roofs formed by the epidermis were carefully removed. Genomic DNA was then extracted using a combination of mechanic grinding using a TissueLyzer II system (Qiagen, Hilden, Germany) and chemical purification on silica columns (DNeasy Blood and Tissue kit, Qiagen). Isolated DNA was then enzymatically hydrolyzed into monomers using a previously reported technique (Douki, 2013). Isotopically labeled (M+12) internal standards were added to hydrolyzed samples before HPLC-MS/MS analysis.
Quantification involved injection of the samples into a chromatographic system consisting of an online solid phase extraction column (C18, 2 × 50 mm) and an UHPLC analytical column. Samples were injected into the online solid phase extraction column and then directed toward the analytical one. A gradient of acetonitrile in triethylammonium acetate was used. The outlet of the chromatographic system was directed toward the inlet of a mass spectrometer used in the electrospray ionization mode. Detection was performed in the specific and sensitive multiple reaction-monitoring mode. Thymine photoproducts were analyzed in the form of dinucleoside monophosphates, and three fragmentation reactions were monitored for each. In DNA, normal nucleosides were also quantified using a UV detector.
Measurement of the creatinine level in urine
Creatinine, a urinary marker reflecting fluctuations in overall excretion from one urine collection and from one donor to the other, was used to normalize the level of photoproducts in the samples between the groups. Creatinine assay was performed using an enzymatic method on the basis of modified kinetic Jaffe technique, and the signal was detected with an automated Dimension Vista System (ECREA assay, Siemens, Munich, Germany). Analyses were done by Laboratoire Biolab Avenir, Clinique Pasteur (Toulouse, France), and the results were expressed in mM creatinine.
Statistical analysis
Data are presented as median with 25th–75th percentiles (quartile 1–quartile 3). Twenty-three subjects (11 in the educated group and 12 in the control group) corresponding to the full analysis set were included in this study. For urinary photoproducts, a covariance analysis explaining the evolution of the different quantitative parameters between visit 1 and visit 2 was performed. The group factor (educated group vs. control group) was included as a fixed factor, and the baseline visit 1 value was included as a covariate. Intragroup and inter-roup comparisons were made on differences of adjusted means calculated by the model. A covariance analysis on the change between visit 1 (before) and visit 2 (after) with group as fixed factor and baseline as the covariate was made. Comparisons between LSMeans (least square means) were realized to perform intragroup and intergroup analyses. This choice to use the change from baseline for analysis is compliant with the guidelines. Moreover, inclusion of the baseline as a covariate followed the recommendations of the European Medicines Agency (2015). For photoproducts in the DNA from suction blisters, a one-way ANOVA explaining the value of the different quantitative parameters at visit 2 was performed with the group factor (educated group vs. control group) as a fixed factor. Analyses were performed with Software SAS 9.4, with P-value significant at 0.05 bilateral test
Ethics statement
In agreement with French law (ordonnance n° 2016-800 June 16, 2016 and decree n° 2017-884 May 9, 2017), this study could not be submitted to an ethics committee. French law considers that studies evaluating cosmetic products do not have to be submitted to an ethics committee. The study was performed in a clinical research center that is accredited by the French National Agency for Safety of Medicinal Products in compliance with the Declaration of Helsinki (1964) and its subsequent revisions and in the spirit of Good Clinical Practice. All volunteers provided written and informed consent. The consent form clearly explained the number and the type of sampling. All samples were anonymized before analysis.
Data availability statement
Datasets related to this article (raw urine and DNA data, description of the volunteers) are available as an Excel file at the following link:
https://figshare.com/articles/dataset/INNOV-2023-0003_data_table_xlsx/23654430
ORCIDs
Thierry Douki: http://orcid.org/0000-0002-5022-071X
S. Caillat: http://orcid.org/0009-0001-8172-6254
D. Bacqueville: http://orcid.org/0000-0001-9091-2772
C. Géniès: http://orcid.org/0000-0003-3535-4530
C. Huyghe: http://orcid.org/0009-0000-8861-3853
H. Duplan: http://orcid.org/0000-0001-9414-8070
J. Le Digabel: http://orcid.org/0000-0003-4970-4466
C. Lauze: http://orcid.org/0000-0001-7295-1241
J. Filiol: http://orcid.org/0000-0002-3506-8633
R. Marinescu: http://orcid.org/0009-0003-6360-3829
K. Bouyer: http://orcid.org/0009-0005-2017-6729
E. Questel: http://orcid.org/0000-0002-4229-7186
Gwendal Josse: http://orcid.org/0000-0003-3375-5226
Conflict of interest
The authors state no conflict of interest.
Acknowledgments
The authors wish to thank Thierry Belleau for performing creatinine assays and Jennyfer Loustau for clinical design. No clinical trial registration is mandatory in France for cosmetic-oriented human studies.
Author Contributions
Conceptualization: TD, KB, EQ, GJ; Investigation: SC, DB, CG, CH, JLD, HD, CL, JF, RM, EQ, GJ; Project Administration: TD, KB, GJ; Validation: TD, KB, GJ; Writing – Original Draft Preparation: TD, CL, EQ, GJ; Writing – Review and Editing: TD, CL, EQ, GJ
accepted manuscript published online XXX; corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2023.100227
References
- Ahmad J., Cooke M.S., Hussieni A., Evans M.D., Patel K., Burd R.M., et al. Urinary thymine dimers and 8-oxo-2'-deoxyguanosine in psoriasis. FEBS Lett. 1999;460:549–553. doi: 10.1016/s0014-5793(99)01402-7. [DOI] [PubMed] [Google Scholar]
- Autier P., Boniol M., Doré J.F. Sunscreen use and increased duration of intentional sun exposure: still a burning issue. Int J Cancer. 2007;121:1–5. doi: 10.1002/ijc.22745. [DOI] [PubMed] [Google Scholar]
- Autier P., Boniol M., Severi G., Doré J.F. European Organizatin for Research and Treatment of Cancer Melanoma Co-operative Group. Quantity of sunscreen used by European students. Br J Dermatol. 2001;144:288–291. doi: 10.1046/j.1365-2133.2001.04016.x. [DOI] [PubMed] [Google Scholar]
- Bacqueville D., Douki T., Duprat L., Rebelo-Moreira S., Guiraud B., Dromigny H., et al. A new hair follicle-derived human epidermal model for the evaluation of sunscreen genoprotection. J Photochem Photobiol B. 2015;151:31–38. doi: 10.1016/j.jphotobiol.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Bacqueville D., Jacques-Jamin C., Dromigny H., Boyer F., Brunel Y., Ferret P.J., et al. Phenylene bis-diphenyltriazine (triAsorB), a new sunfilter protecting the skin against both UVB + UVA and blue light radiations. Photochem Photobiol Sci. 2021;20:1475–1486. doi: 10.1007/s43630-021-00114-x. [DOI] [PubMed] [Google Scholar]
- Bacqueville D., Jacques-Jamin C., Lapalud P., Douki T., Roullet N., Sereno J., et al. Formulation of a new broad-spectrum UVB + UVA and blue light SPF50+ sunscreen containing phenylene bis-diphenyltriazine (triAsorB), an innovative sun filter with unique optical properties. J Eur Acad Dermatol Venereol. 2022;36:29–37. doi: 10.1111/jdv.18196. [DOI] [PubMed] [Google Scholar]
- Bonilla X., Parmentier L., King B., Bezrukov F., Kaya G., Zoete V., et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48:398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- Cadet J., Douki T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem Photobiol Sci. 2018;17:1816–1841. doi: 10.1039/c7pp00395a. [DOI] [PubMed] [Google Scholar]
- Chen Q., Li R., Zhao X., Liang B., Ma S., Li Z., et al. Prevention of ultraviolet radiation-induced immunosuppression by sunscreen in Candida albicans-induced delayed-type hypersensitivity. Mol Med Rep. 2016;14:202–208. doi: 10.3892/mmr.2016.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M.S., Evans M.D., Burd R.M., Patel K., Barnard A., Lunec J., et al. Induction and excretion of ultraviolet-induced 8-oxo-2'-deoxyguanosine and thymine dimers in vivo: implications for PUVA. J Invest Dermatol. 2001;116:281–285. doi: 10.1046/j.1523-1747.2001.01251.x. [DOI] [PubMed] [Google Scholar]
- Douki T. The variety of UV-induced pyrimidine dimeric photoproducts in DNA as shown by chromatographic quantification methods. Photochem Photobiol Sci. 2013;12:1286–1302. doi: 10.1039/c3pp25451h. [DOI] [PubMed] [Google Scholar]
- Durinck S., Ho C., Wang N.J., Liao W., Jakkula L.R., Collisson E.A., et al. Temporal dissection of tumorigenesis in primary cancers. Cancer Discov. 2011;1:137–143. doi: 10.1158/2159-8290.CD-11-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghissassi F., Baan R., Straif K., Grosse Y., Secretan B., Bouvard V., et al. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency . 2015. Guideline on adjustment for baseline covariates in clinical trials.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-adjustment-baseline-covariates-clinical-trials_en.pdf; (accessed November 15, 2021) [Google Scholar]
- Freeman S.E., Ley R.D., Ley K.D. Sunscreen protection against UV-induced pyrimidine dimers in DNA of human skin in situ. Photodermatol. 1988;5:243–247. [PubMed] [Google Scholar]
- Green A., Williams G., Neale R., Hart V., Leslie D., Parsons P., et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial [published correction appears in Lancet 1999;354:1038] Lancet. 1999;354:723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- Green A.C., Williams G.M., Logan V., Strutton G.M. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- Hayward N.K., Wilmott J.S., Waddell N., Johansson P.A., Field M.A., Nones K., et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- Hodis E., Watson I.R., Kryukov G.V., Arold S.T., Imielinski M., Theurillat J.P., et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Standardization Organization . 2019. ISO 24442:2011: cosmetics — sun protection test methods — in vivo determination of the suncreen UVA protection.https://www.iso.org/standard/46521.html; (accessed September 28, 2023) [Google Scholar]
- Josse G., Douki T., Le Digabel J., Gravier E. The use of suction blisters to measure sunscreen protection against UVR-induced DNA damage. J Photochem Photobiol B. 2018;179:1–6. doi: 10.1016/j.jphotobiol.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Josse G., Gravier E., Douki T. Method for the accurate determination of the DNA protection factor of sun protection products. Br J Dermatol. 2020;183:178–179. doi: 10.1111/bjd.18936. [DOI] [PubMed] [Google Scholar]
- Kciuk M., Marciniak B., Mojzych M., Kontek R. Focus on UV-induced DNA Damage and repair-disease relevance and protective strategies. Int J Mol Sci. 2020;21:33. doi: 10.3390/ijms21197264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotova N., Hemminki K., Segerbäck D. Urinary thymidine dimer as a marker of total body burden of UV-inflicted DNA damage in humans. Cancer Epidemiol Biomarkers Prev. 2005;14:2868–2872. doi: 10.1158/1055-9965.EPI-05-0164. [DOI] [PubMed] [Google Scholar]
- Le Curieux F., Hemminki K. Cyclobutane thymidine dimers are present in human urine following sun exposure: quantitation using 32p-postlabeling and high-performance liquid chromatography. J Invest Dermatol. 2001;117:263–268. doi: 10.1046/j.1523-1747.2001.01416.x. [DOI] [PubMed] [Google Scholar]
- Lerche C.M., Philipsen P.A., Hermansson S., Heydenreich J., Wulf H.C. Quantification of urinary thymidine dimers in volunteers after ultraviolet radiation using a new UPLC-MS/MS-based method. Anticancer Res. 2022;42:5069–5076. doi: 10.21873/anticanres.16015. [DOI] [PubMed] [Google Scholar]
- Moyal D.D., Fourtanier A.M. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol. 2008;58:S149–S154. doi: 10.1016/j.jaad.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Narayanan D.L., Saladi R.N., Fox J.L. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- Neale R., Williams G., Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol. 2002;138:1319–1325. doi: 10.1001/archderm.138.10.1319. [DOI] [PubMed] [Google Scholar]
- Olsen C.M., Wilson L.F., Green A.C., Biswas N., Loyalka J., Whiteman D.C. Prevention of DNA damage in human skin by topical sunscreens. Photodermatol Photoimmunol Photomed. 2017;33:135–142. doi: 10.1111/phpp.12298. [DOI] [PubMed] [Google Scholar]
- Paul S.P. Ensuring the safety of sunscreens, and their efficacy in preventing skin cancers: challenges and controversies for clinicians, formulators, and regulators. Front Med (Lausanne) 2019;6:195. doi: 10.3389/fmed.2019.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B., Datta P., Philipsen P.A., Wulf H.C. Sunscreen use and failures--on site observations on a sun-holiday. Photochem Photobiol Sci. 2013;12:190–196. doi: 10.1039/c2pp25127b. [DOI] [PubMed] [Google Scholar]
- Petersen B., Wulf H.C., Triguero-Mas M., Philipsen P.A., Thieden E., Olsen P., et al. Sun and ski holidays improve vitamin D Status, but are associated with high levels of DNA damage. J Invest Dermatol. 2014;134:2806–2813. doi: 10.1038/jid.2014.223. [DOI] [PubMed] [Google Scholar]
- Reynaud N., Belz L., Béal D., Bacqueville D., Duplan H., Géniès C., et al. DNA photoproducts released by repair in biological fluids as biomarkers of the genotoxicity of UV radiation. Anal Bioanal Chem. 2022;414:7705–7720. doi: 10.1007/s00216-022-04302-1. [DOI] [PubMed] [Google Scholar]
- Rueegg C.S., Stenehjem J.S., Egger M., Ghiasvand R., Cho E., Lund E., et al. Challenges in assessing the sunscreen-melanoma association. Int J Cancer. 2019;144:2651–2668. doi: 10.1002/ijc.31997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E.S.D., Tavares R., Paulitsch F.D.S., Zhang L. Use of sunscreen and risk of melanoma and non-melanoma skin cancer: a systematic review and meta-analysis. Eur J Dermatol. 2018;28:186–201. doi: 10.1684/ejd.2018.3251. [DOI] [PubMed] [Google Scholar]
- van Praag M.C., Roza L., Boom B.W., Out-Luijting C., Henegouwen J.B., Vermeer B.J., et al. Determination of the photoprotective efficacy of a topical sunscreen against UVB-induced DNA damage in human epidermis. J Photochem Photobiol B. 1993;19:129–134. doi: 10.1016/1011-1344(93)87107-x. [DOI] [PubMed] [Google Scholar]
- Young A.R., Greenaway J., Harrison G.I., Lawrence K.P., Sarkany R., Douki T., et al. Sub-optimal application of a high SPF sunscreen prevents Epider-mal DNA damage in vivo. Acta Derm Venereol. 2018;10:880–887. doi: 10.2340/00015555-2992. [DOI] [PubMed] [Google Scholar]
- Young A.R., Sheehan J.M., Chadwick C.A., Potten C.S. Protection by ultraviolet A and B sunscreens against in situ dipyrimidine photolesions in human epidermis is comparable to protection against sunburn. J Invest Dermatol. 2000;115:37–41. doi: 10.1046/j.1523-1747.2000.00012.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets related to this article (raw urine and DNA data, description of the volunteers) are available as an Excel file at the following link:
https://figshare.com/articles/dataset/INNOV-2023-0003_data_table_xlsx/23654430




