Abstract
Nanomedicine is a critical therapeutic approach for treating most poultry illnesses, particularly parasitic infections. Coccidiosis is a severe protozoan infection affecting poultry; the emergence of drug-resistant Eimeria strains demands the development of new, safe therapies. Consequently, the objective of this work was to investigate the efficacy of the biosynthesized selenium nanoparticles (SeNPs) by Paenibacillus polymyxa (P. polymyxa) against Eimeria tenella (E. tenella) experimental infection in broiler chickens. The prepared SeNPs absorbed the UV at 270 nm were spherical with a size of 26 nm, and had a surface negative charge of −25 mV. One hundred and fifty, 1-day-old male broiler chicks were randomly allocated into 5 groups (30 birds/group with triplicates each) as follows: T1: negative control (noninfected and nontreated with SeNPs); T2: delivered SeNPs (500 µg/kg diet) for 35 successive days, T3: E. tenella-infected (positive control birds), T4: E. tenella-infected and treated with SeNPs (500 µg/kg diet) and T5: E. tenella-infected chicks and treated with anticoccidial agent (sulfadimidine, 16% solution 8 mL/L of drinking water) for 5 successive days. At 14 d of age, each bird in infected groups was orally treated with 3 × 103 sporulated oocyst of E. tenella. SeNPs considerably decreased the number of oocysts in broiler feces compared to positive control and anticoccidial drug, followed by a substantial reduction of parasite phase count in the cecum (15, 10, and 8 for meronts, gamonts, and developing oocysts) when compared with positive control birds. The Eimeria experimental infection lowered the activity of antioxidant enzymes, superoxide dismutase (SOD), glutathione peroxidase (GPx), and reduced glutathione (GSH) while increasing the stress parameters nitric oxide (NO) and malonaldehyde (MDA). Moreover, the production of proinflammatory (TNF-α and IL-6) and apoptotic genes (BcL2 and Cas-3) were significantly elevated. Administrating SeNPs to chicks significantly decreased oxidative stress, inflammation, and apoptotic markers in the cecum tissue. Therefore, growth performance, carcass weights, antioxidant enzymes, and blood properties of infected chicks were enhanced. The findings compared the protecting role of Se-nanoparticles against cecum damages in E. tenella-infected broilers.
Key words: anticoccidial, bacteriogenic selenium nanoparticle, broiler, Eimeria, immunity
INTRODUCTION
The poultry sector is a major economic driver worldwide, as the demand for safe and high-protein poultry products continues to grow in tandem with the world's rapidly expanding population but faces many adverse problems (Swelum et al., 2021; Abd El-Hack et al., 2022). Coccidiosis is a significant expense for broiler chicks, ranging between $3.34 and $3.89 per m2 of broiler house if not managed (Gilbert et al., 2020; El-Maddawy et al., 2022). Coccidiosis induces severe depression in birds and is characterized clinically by a variable degree of diarrhea, which may reach hemorrhagic type; it reflects poorly on birds' productivity as it induces destruction in the intestinal villi and interferes with nutrients absorption and metabolism (El-Shall et al., 2022). Since 1948, different chemical anticoccidial drugs have been applied to treat coccidial infections, including ionophores antibiotics in the 1970s (Chapman, 2018).
Due to haphazard treatment resistance, coccidial endemics persist despite this (El-Shall et al., 2022). Inappropriately, no new drugs have been licensed for usage, which has heightened the desire for innovative anticoccidial options derived from nature (Noack et al., 2019).
Recently, nanotechnology has been applied in the veterinary sector for disease diagnosis, developing medicines and preventatives, and nanoparticle manufacturing has increased in tandem with demand therapies (El-Maddawy et al., 2022). Chemical approaches include (N, N-dimethyl formamide, sodium dodecyl sulfate, sodium borohydride, and sodium citrate) and physical (cluster beam condensation or laser ablation, sputter deposition, and thin films), they are employed for nanoparticle production (El-Ashry et al., 2022). These techniques are costly and environmentally hostile (Saad et al., 2022).
Therefore, the synthesis of green nanoparticles using natural stabilizers (plants, bacteria, macroalgae, yeast, and fungus) is highly promising; adding a biological reducing agent makes the product cheaper to produce, more accessible, and less hazardous (El-Saadony et al., 2019, 2021a; Abdel-Moneim et al., 2022). Both conventional and biologically synthesized nanoparticles are stable (El-Saadony et al., 2021b). These characteristics feature antibacterial action (Abdel-Moneim et al., 2022). This is especially crucial in light of the rising multiresistance of pathogenic and conditionally harmful bacteria, such as E. tenella, Pseudomonas aeruginosa, and Escherichia coli, which have developed a high level of antibiotic resistance (Han et al., 2021; Salem et al., 2022b).
The nanoparticles are advantageous for biological applications due to their diminutive size and distinct physicochemical characteristics; they provide regulated drug administration, release, and in vivo immunomodulation (Yousry et al., 2020). Elemental nanoparticles possess antimicrobial, antiparasitic, and free radicals scavenging activities (Bisht et al., 2022; Arafa et al., 2023).
New antiparasitic agents include nanotechnological methods and other fresh developments (Sarhan et al., 2022). Nanoparticles are interesting medicines for treating several parasitic disorders due to their unique features, different from those of solitary atoms and bulk material (Carvalho et al., 2020; Attia et al., 2022). Selenium nanoparticles were produced in this study utilizing P. polymyxa MS 411; P. polymyxa is a G+ spore-forming rhizobacterium that generates EPSs, enzymes, plant hormones, and antibiotics (Rybakova et al., 2016).
For several significant P. polymyxa strains, whole genome sequences have been reported that code for producing various physiologically active chemicals (Liu et al., 2017) with very diversified physiological and biotechnological roles and little toxicity (Liang and Wang, 2015). Nanoselenium provides several health advantages, such as anticoccidial, antioxidant, and anti-inflammation activities (Prokisch and Zommara, 2011; Alkhudhayri et al., 2020). In addition, SeNPs have been found to have potential activities against various parasites such as murine schistosomiasis, giardiasis, and murine trichinosis (Dkhil et al., 2016; Malekifard et al., 2020; Sarhan et al., 2022).
This study's purpose was to assess the protective impact of biosynthesized SeNPs using P. polymyxa MS 411 against experimental infection with E. tenella in broiler chickens and then to examine the impact of bacterial SeNPs supplementation on the performance, carcass, and blood properties of infected chicks.
MATERIALS AND METHODS
Isolation and Identification of SeNPs-Producing Bacteria; Fabrication and Characterization of Bacterial Se Nanoparticles
Paenibacillus polymyxa MS 411 strain was used to fabricate green selenium nanoparticles (SeNPs). This strain was isolated from corn soil rhizosphere. The soil samples were collected, mixed, and transferred to the microbiology laboratory. Serial dilutions from soil samples were prepared as follows: 10 g soil was homogenized in 90 mL peptone water to obtain 10−1 dilution, 1 mL from the previous dilution was added to 9 mL of peptone water to get 10−2 dilution, and serial dilutions up to 10−7. 100 µL of each dilution was inoculated on Luria-Bertani (LB) agar plates supplemented with 1, 2, 3, 4, and 5 mM sodium selenite (Na2SeO3) in sterilized Petri plates (90-mm diameter) and then incubated at 30°C for 2 d. The red colonies were chosen according to their ability to reduce sodium selenite (Saad et al., 2022).
Hundred microliters of the MS 411 isolate was inoculated in 100 mL of LB broth and incubated in a shaking incubator (220 rpm, 30°C) for 2 d. The mixture was centrifuged at 6,800 × g for 25 min; The supernatant was discarded and the bacterial pellets were collected then homogenized in 100 mL of the enrichment medium (EM) broth containing 4 mM sodium selenite. The mixture was then incubated in shaking incubator (30°C, 220 rpm) for 2 d. After the incubation time, the change in color of the flask (EM medium containing sodium selenite and the MS 411 isolate) from brilliant yellow to red demonstrates the reduction of sodium selenite to SeNPs by MS 411 isolate. The bacterial SeNPs were extracted from the MS 411 isolates pellets through autoclaving until the explosion of the bacterial membrane; the resulting solution containing bacterial SeNPs was centrifuged at 12,000 × g for 20 min, the supernatant was subsequently obtained, and the exploded cells were discarded. The selenium-producing bacterium was initially identified by morphological and biochemical characteristics, which were then compared to the reference standards in the Bergey's manual. Further identification was conducted by MALDI-TOF spectroscopy; the selected isolate Paenibacillus polymyxa MS411 was identified as Paenibacillus polymyxa DSM 365 (El-Saadony et al., 2021b; Saad et al., 2022; Alowaiesh et al., 2023). Transmission electron microscopy (TEM) studies shape, and aggregation. Dynamic light scattering (DLS) determine the particle size distribution, and zeta potential was used to determine surface charge stability (Abdel-Moneim et al., 2022; Alowaiesh et al., 2023).
Eimeria spp., Isolation, and Identification
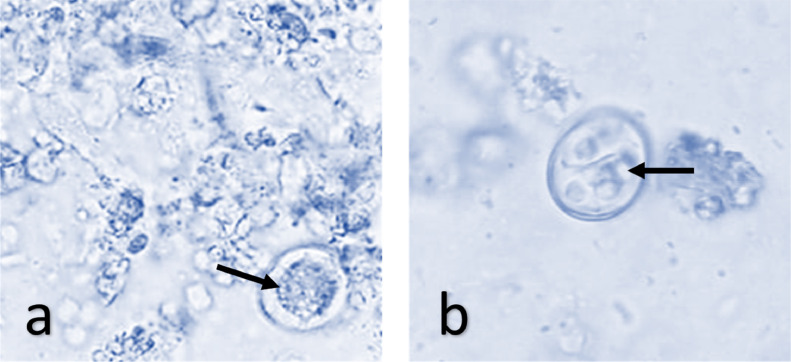
Oocysts utilized in this investigation were isolated from the fresh feces of naturally infected chickens; the identification of the species was based on the morphological descriptions as mentioned by Thienpont et al. (1986); the oocysts were isolated and concentrated by flotation in a salt-saturated solution. The acquired suspension of unsprouted oocysts was combined with 2.5% with an equal amount of potassium dichromate to produce the stock oocyst suspension (OS) (Wiedosari and Wardhana, 2018) that incubated at 27°C for 3 d. Then, the sporulated oocysts were orally inoculated in 5 healthy chickens (2-wk old) to propagate the oocysts, and at 7-days postinfection, the birds were ethically euthanized. Cecal contents were collected, then concentrated, and oocysts were sporulated and kept in a refrigerator until use (Davies et al., 1963). The McMaster technique determined the oocysts count in (mL). At 14 d of age, each bird was orally treated with 3 × 103 sporulated oocyst of E. tenella using oral gavage (Habibi et al., 2016). The shape of sporulated oocysts was used to identify isolated Eimeria species (Figure 1).
Figure 1.
Morphology of E. tenella oocyst: (A) Unsporulated oocyst; (B) sporulated oocyst.
In Vitro Assessment of the Anticoccidial Activity of SeNPs Against E. Tenella
The anticoccidial activity of SeNPs at concentrations (100, 300, 500, 700, and 900 µg) was in vitro evaluated compared with anticoccidial drugs (ACD, sulfadimidine, 16%). All treatments were tested with triplicates, each against E. tenella oocytes, by adding 100 µL of tested nanoparticles to 5 mL of OS (unsporulated oocyst), then incubated at 27°C for 3 d and calculating the number of sporulated oocysts by The McMaster method and capturing microphotographs with an Olympus camera (Tokyo, Japan) (Salem et al., 2022a).
Experimental Design
One hundred and fifty, 1-day-old Cobb male broiler chicks were grown for 5 wk. The birds were reared in separate floor pens with straw bedding on the concrete floor in adjusted environmental conditions with suitable lighting programs, temperature, and humidity according to the birds’ requirements. The illumination system was 23 h light:1 h dark for the first 3 d, then 20 h light:4 h dark until the end of the experiment. Random assignment of broiler chicks to 5 treatment groups (30 birds/group), each with triplicates (10 birds per replicate), as shown in Table 1. In group 1 (T1), birds were fed commercial balanced diets [starter (1–14-days old), grower (15–28-days old), and finisher (29–35-days old)] without any anticoccidial or anticlostridial drugs and supplied with clean, clear water ad libitum. The temp was set at 35°C at the beginning of bird rearing, then decreased by 2°C to 5°C every week to adjust as 22°C at the end of the fourth week; the temperature was then maintained at 22°C for the fifth week. Based on a preliminary experiment, group 2 (T2) was continuously supplemented with SeNPs (500 µg/kg diet) for 35 d. Group 3 (T3) E. tenella-infected chicks, Group 4 (T4), E. tenella-infected chicks and then treated with SeNPs (500 µg/kg diet) based on in vitro study. Group 5 (T5), E. tenella-infected chicks and then treated with the anticoccidial agent (sulfadimidine, 16% solution 8 mL/L of drinking water) (Hunduma and Kebede, 2016). After 4 d from experimental infection, treatments in T4 and T5 were added (continuous 24 h) in feed and water for 5 successive days.
Table 2.
Primers used for the gene encoding the RNAs for some selected proteins (Salem et al., 2022).
| Gene | Primer sequence (5′→3′) |
|---|---|
| B cell leukemia/lymphoma 2 (Bcl-2) | F AGCATGCGACCTCTGTTTGA |
| R GCCACACGTTTCTTGGCAAT | |
| Caspase-3 (Casp3) | F GGGGAGCTTGGAACGCTAAG |
| R CCACTGACTTGCTCCCATGT | |
| Tumor necrosis factor-alpha (TNF-α) | F ACCCTCACACTCACAAACCA |
| R ACCCTGAGCCATAATCCCCT | |
| Interleukin 6 (IL-6) | F CTGCAAGAGACTTCCATCCAG |
| R AGTGGTATAGACAGGTCTGTTGG | |
| β-Actin (ACTB) | F CACCATTGGCAATGAGCGGTTC |
| R AGGTCTTTGCGGATGTCCACGT |
Fecal samples were collected from all experimental groups at 4-, 7-, 10-, and 14-days postinfection to estimate oocyst shedding using the McMaster technique to determine the oocyst count per gram of freshly voided droppings.
The experimental room was always accessible under the same environmental circumstances. At specified intervals, birds were vaccinated against common viruses [avian influenza (AI), Newcastle disease (ND), infectious bronchitis (IB), infectious bursal disease (IBD)].
Growth Performance
Each day, the mortality rate of the chicks was evaluated. On the first day, they were weighed separately and then weekly. The chickens' weight increase (g), feed consumption, and feed conversion ratio (FCR) were then measured.
where: Total weight of product = final weight − starting weight.
Carcass Traits
At the end of the experiment, 10 chicks were randomly selected from each treatment and ethically slaughtered to assess the hot carcass weight, gizzard, heart, liver, and visceral fat.
Biochemistry of Blood
At the time of slaughter, blood samples were collected from 10 birds per group, centrifuged at 4,500 × g for 20 min, and the plasma was stored at 20°C. Using commercial kits provided by the biodiagnostic company, El-Tahrer St., Dokki, Giza, Egypt, total protein (CAT. NO. TB2020), albumin (CAT. No. AB 10 10), AST, ALT (CAT. NO. AP 1020), GSH, MDA (CAT. No. MD 25 29), SOD, GPx, and NO (CAT. No. CA 25 17) were evaluated calorimetrically according to the instructions provided by the manufacturer (Alagawany et al., 2022).
Blood Hematology
At the end of the experiment, blood samples were collected from 10 birds per group; red blood cells (RBCs), platelet cells (PBCs), and white blood cells (WBCs) were estimated immediately after blood was drawn and heparinized (Alagawany et al., 2021). On clean microscope slides, thin blood smears were created from each sample. They were air-dried before the slides were stained with a modified form of Wright's stain. Under 100 lens power, 100 cells were tallied (Alagawany et al., 2021).
Gut Microbiota
Intestinal content from each treatment's cecum was collected separately in sterile glass flasks after slaughter. Digesta were evacuated and mixed. Flasks were kept at 4°C till the determination of microbial counts. Ten-fold serial dilutions of up to 10−7 of each sample were prepared. Total bacterial counts, yeasts and molds count, Escherichia coli, and lactobacilli count were estimated. A nutrient agar medium was used to enumerate aerobic bacteria (Amadi and Wami, 2023). Sabouraud dextrose agar (SDA) was used to count yeasts and molds (Alagawany et al., 2021). The eosin methylene blue (EMB) agar medium (Oxoid) was used for Escherichia coli counts. The deMan, Rogosaand Sharpe (MRS) agar medium was used for lactobacilli count. After incubation, colonies were counted. Numbers of colony-forming units (CFU) are expressed as log CFU per gram of cecal content.
RNA Isolation
Ten days after the experimental infection, 2 birds per replicate were ethically slaughtered, and then cecal tissues were hygienically dissected. The samples were collected and homogenized for 30 s at 35,000 oscillations/min in a Mini Bead-beater 24 (Bio-Spec, Bartlesville, OK) and chilled on ice, then extracted using an RNA separation kit (Ambion, Applied Biosystems, Darmstadt, Germany). Later, total RNA was isolated from preserved cecal tissues using Trizol (Invitrogentham, MA). Following the manufacturer's recommendations, RNA samples were digested for at least 1 h with DNase (Applied Biosystems, Darmstadt, Germany) before being transcribed into cDNA using the reverse transcription kit (Qiagen, Hilden, Germany). The QuantiTectTM SYBR Green PCR kit (Qiagen) performing the real-time PCR according to the manufacturer's instructions. The TaqMan 7500 system software quantifying the amplification data. Primers were used to target the genes encoding the mRNAs for caspase-3 (Casp3), B cell leukemia/lymphoma 2 (BcL2), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). The results are shown in Table 2. The PCR techniques were identical to those previously detailed by Dkhil et al. (2013). The Ct (ΔΔCT) approach outlined by Livak and Schmittgen (2001) measured the variances between the mean gene expression and the reference gene. The qRT-PCR was done 3 times on each sample. In a 40-cycle PCR amplification, the cycling conditions were denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 45 s.
Table 1.
Experimental groups of infected and noninfected broiler.
| Groups | Treatments |
|---|---|
| T1 | Non infected chicks |
| T2 | Non infected chicks + SeNPs 500 µg/kg diet for 35 d |
| T3 | E. tenella-infected chicks and non treated |
| T4 | E. tenella-infected chicks + treated with SeNPs 500 µg/kg diet for 5 d |
| T5 | E. tenella-infected chicks + treated with sulfadimidine, 16% solution 8 mL/L of drinking water for 5 d |
Histopathological Studies
At 10-days postexperimental infection, 2 birds were ethically slaughtered per replicate, and then cecal tissues were collected. According to Adam and Caihak (1964), paraffin cecal tissue slices 5-µm thick were produced for staining with H&E for further histopathological examination. We counted meronts, gamonts, and developing oocysts in infected and infected-treated groups to distinguish between the various parasite stages in the cecum of birds (Yang et al., 2022). The values were represented in units of 10 villous crypts (VCU).
Statistical Analysis
Prior to conducting a 1-way ANOVA, pretests were administered. The assumption of normality for sample distributions were examined and found P values of 0.0001 and Levene test for homogeneity revealed P value of 0.015. The means of the triplicate data were evaluated for significant differences using 1-way ANOVA with a 95% level of confidence (Wallenstein et al., 1980), using SAS software (version 9.4, Cary, NC, 2020). The sample size was calculated from the following equation
Means were compared with the least significant difference (LSD) as a post hoc test at a probability level of 5%.
RESULTS
SeNPs Characterization
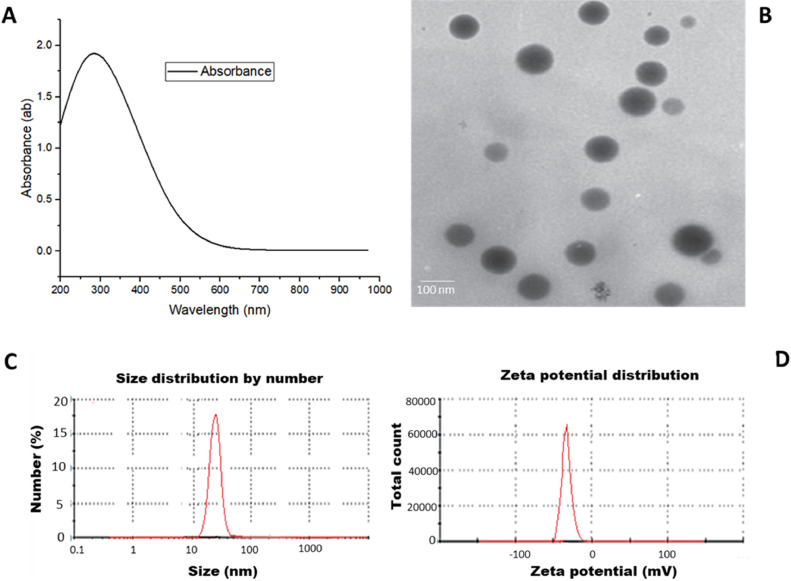
The Paenibacillus polymyxa successfully converted the sodium selenite to selenium nanoparticles, indicating color conversion from colorless to red. The bacterial SeNPs absorbed the UV at 270 nm (Figure 2A); their shape was spherical (Figure 2B) with an average diameter of 26 nm measured by zeta sizer and a negative charge of −25 mV (Figure 2C and D) by zeta potential analysis.
Figure 2.
Physiochemical characterization of SeNPs produced by P. polymyxa MS 411. (A) color conversion measured by UV at 270, (B) the shape of nanoparticles by TEM, (C) the size of nanoparticles by zeta sizer, (D) the charge of nanoparticles by zeta potential.
Output of Developing Oocysts Under Selenium Nanoparticles Treatment
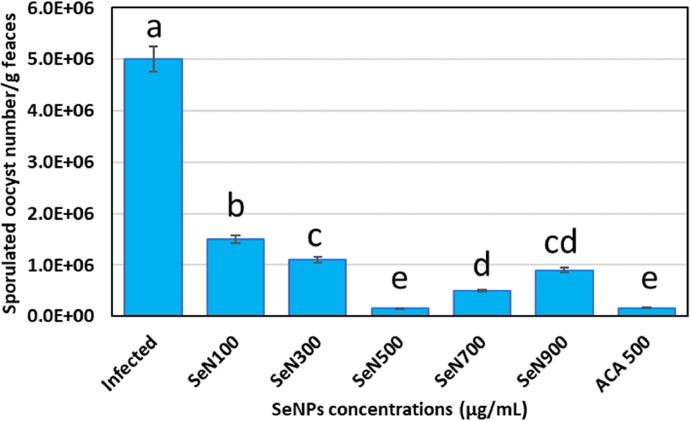
Figure 3 shows the considerable anticoccidial activity of SeNPs at concentrations (100, 300, 500, 700, and 900 µg/mL) compared to anticoccidial drugs (ACD, sulfadimidine, 16%). The results showed that SeNPs reduced the Eimeria oocyst count by 70 to 97% based on the concentrations; the count of sporulated oocytes in control was 5,000,000; at the best SeN concentration of 500 µg/mL, it was reduced to 150,000 as a 97% reduction. The best concentration was 500 µg/mL head-to-head with the anticoccidial drug; therefore, this concentration was during the in vivo experiment.
Figure 3.
Preliminary in vitro experiment for determining the best selenium nanoparticles concentration (SeN) against E. tenella showing that SeNPs with a concentration of (500 µg/mL) as well as ACD (sulfadimidine as anticoccidial drug) significantly reduced E. tenella oocysts sporulation. The values are presented as mean ± SE, lowercase letters (a–e) above columns indicate the significant differences between different treatments on the sporulated oocyst count using LSD test at P < 0.05.
T4 and T5 revealed a significant reduction in oocysts shedding number per gram droppings in all checking points (4-, 7-, 10-, 14-days postinfection) in contrast with T3.
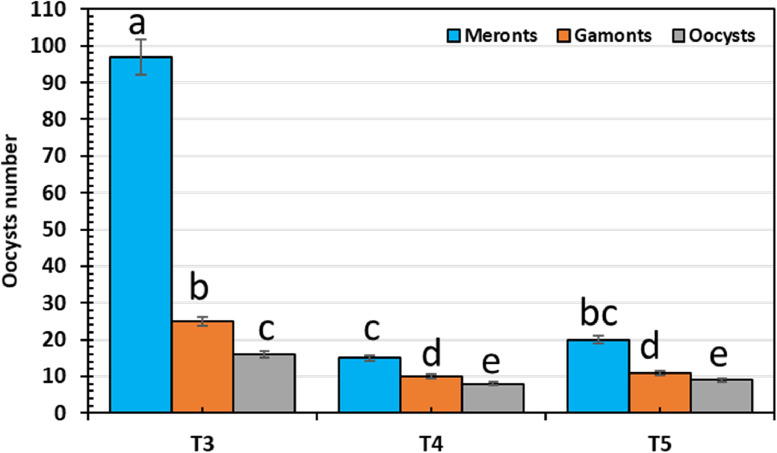
The life cycle of E. tenella was developed from meronts and gamonts to developing oocysts in the cecal epithelial cells of infected broiler chickens (Figure 4). In the infected broiler's cecal villi, these phases were recorded per 10 VCUs as 97 meronts, 25 gamonts, and 15 developing oocysts (Figure 4). Remarkably, these numbers were significantly reduced by 85, 60, and 50% in the SeNPs-treated broiler (15, 10, and 8 for meronts, gamonts, and developing oocysts) compared to the anticoccidial drug (20, 11, and 9 for meronts, gamonts, and developing oocysts) the SeNPs is more effective (P < 0.001) than the anticoccidial drug.
Figure 4.
The number of E. tenella developmental stages (meronts, gamonts, and oocysts) in the cecum of infested and treated groups. T3: E. tenella-infected (positive control birds), T4: E. tenella-infected and treated with SeNPs (500 µg/kg diet), and T5: E. tenella-infected chicks and treated with the anticoccidial agent (sulfadimidine, 16% solution 8 mL/L of drinking water). The values are presented as mean ± SE. Lowercase letters (a–e) above columns indicate the significant differences between different treatments on the count of oocyst stages using the LSD test at P < 0.05.
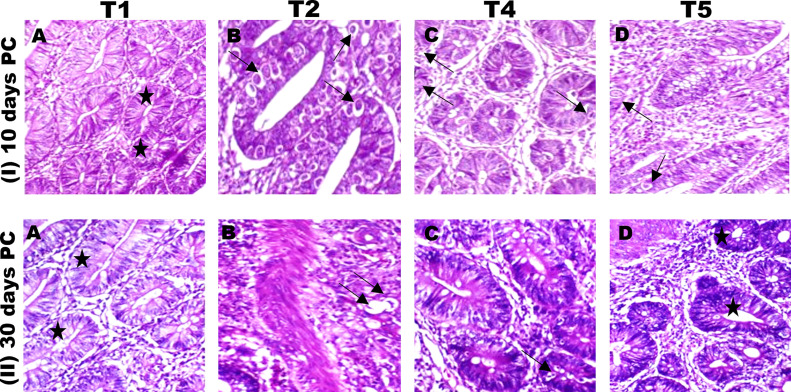
Histological Studies on In Vivo Anticoccidial Effects of SeNPs 10 and 30-Days Postinfection
Figure 5I describes the treatment of 2 groups of infected birds with SeNPs (T4) and sulfadimidine (T5) 10-days postinfection. A) The cecum of a T1 chicken possesses typical intestinal crypts. B) Cecum of a T2 chicken with extensive coccidial infection of the intestinal crypts (black arrows). C) T4 (SeNPs 500 µg/kg) chicken cecum indicating restoration of normal intestinal crypts with few parasite stages (white arrow) and a considerable reduction in the number of afflicted crypts carrying macrogametes of the parasite (black arrow). D) The cecum of T5 chicken exhibits a considerable decrease in the macrogametes of E. tenella (black arrow).
Figure 5.
(I) Histopathology of cecal tissues of chicks infected with E. tenella 10-days postexperimental infection showing (A) normal cecal tissue (star), (B) large number of developmental stages (arrow) (schizonts) of E. tenella in lamina propria of villi, (C and D) reduced number of developmental stages (arrow) (schizonts) of E. tenella in lamina propria of villi in the treated groups. (II) Histopathology of cecal tissues of chicks infected with E. tenella 30-days postexperimental infection showing (A) the cecum of a T1 (control) chicken with typical intestinal crypts (H&E, 200). (B) Cecum of T2 E. tenella- infected chicken and non treated, chicken displaying observable invasion of coccidial schizonts of E. tenella inside the intestinal crypts (arrows), as well as infestation of the lamina propria with various coccidial stages of E. tenella that were mostly degenerated from the inside (arrow) and eosinophilic cells infiltration (arrowhead, H&E, 200, T3 (Non infected chicks + SeNPs) showed also typical intestinal crypts as control; therefore we didn't add the image in figure 5 as the 100% similarity with control). (C) cecum of T4 (E. tenella-infected chicken and treated with SeNPs), chicken cecal tissues displayed restoration of normal intestinal crypts (arrows, H&E, 200). Cecum of T5 (sulfadimidine) chicken displaying a substantial decrease of E. tenella reproducing schizonts, evident hypertrophy of the crypts lining epithelium, and modest interstitial mononuclear cell infiltration (H&E, 200).
Figure 5II depicts the treatment of 2 groups of birds with SeNPs (T4) and sulfadimidine (T5) 30-days postinfection. A) the cecum of a T1 chicken with regular duodenal crypts. B) Cecum of T2 chickens with evident coccidial schizont invasion of E. tenella within the intestinal crypts (arrows). C) Hyperplasia of the crypts lining the epithelial and invasion of inter mononuclear cells in the cecum of T4 (SeNPs) chickens (arrows), cecum of T5 (sulfadimidine) chicken displaying a substantial decrease of E. tenella reproducing schizonts, apparent hypertrophy of the crypts lining epithelium, and modest interstitial mononuclear cell infiltration. This histological examination revealed the curative impact of SeNPs on Eimeria development and alterations in the chicken cecum.
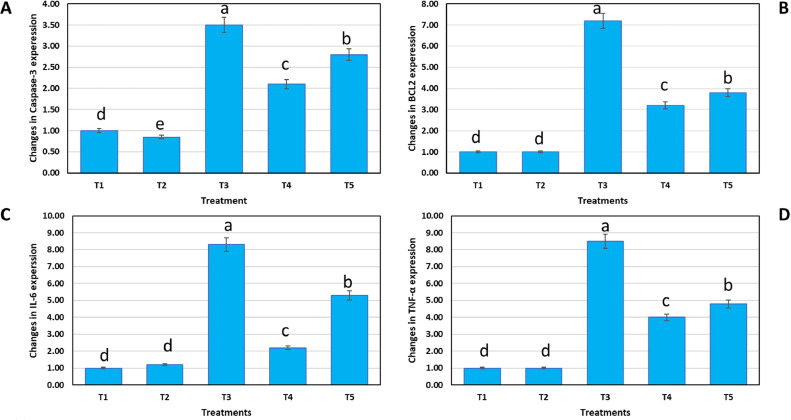
Effect of SeNPs on RNA Expression
The gene expression of BcL-2 and caspase-3 was raised due to an infection with E. tenella (Figure 6A). After treatment with SeNPs, the expression of the cas-3 gene was drastically reduced from 3.5- to 2.0-fold and 2.8-fold in anticoccidial drug. BcL-2 expression was dysregulated from 7.2- to 3.2-fold, as seen in SeNPs-treated groups and 3.8-fold in anticoccidial drug-treated groups (Figure 6B). Due to E. tenella infection, the expression of proinflammatory genes, TNF-α and IL-6, was upregulated in Figure 6C and D. Compared to T1, the expression of these genes increased by around 8.3 and 8.5, respectively. The therapy with Se-nanoparticles (500 µg/L) significantly decreased IL-6 and TNF-α by 2.2- and 4-fold compared to the anticoccidial medication and the control.
Figure 6.
Effect of SeNPs on the RNA expression of caspase-3 (A), BcL-2 (B), IL-6 (C), and TNF-α (D) in the cecum of E. tenella-infected broiler detected by RT-PCR analysis. The values are presented as mean ± SE. Lowercase letters (a–e) above columns indicate the significant differences between different treatments on the proinflammatory markers using the LSD test at P < 0.05. T1: Non infected chicks; T2: Non infected chicks + SeNPs 500 mg/kg diet for 35 d; T3: E. tenella-infected chicks and non-treated; T4: E. tenella-infected chicks + treated with SeNPs 500 mg/kg; diet for 5 d; T5: E. tenella-infected chicks + treated with sulfadimidine, 16% solution 8 mL/L of drinking water for 5 d.
Growth Performance
Table 3 shows the positive impact of SeNPs on the growth performance of noninfected (T2) and E. tenella-infected-treated broilers (T4) compared to control-positive birds in T3 and sulfadimidine-treated birds in T5. The initial BW of chicks did not change substantially from that of the control group, nor was a significant difference across groups. The supplementation of SeNPs dramatically increased their body weight gain, whereas SeNPs supplemented group increased the BWG by 12% compared to the noninfected control; also, SeNPs had anticoccidial activity against E. tenella, where the BWG increased by 5% compared to the anticoccidial drug. Concerning the feed intake, the chicks supplemented with SeNPs had the lowest amount in noninfected groups, but in infected groups, the FI was reduced by 15% in SeNPs-treated groups. Similarly, the FCR was significantly improved in SeNPs-treated birds T4 and T2 followed by T5 when compared with T3 and even T1. Only 20% (6/30) cumulative mortalities were recorded in T3, and no mortalities have been reported in the rest of the experimental groups.
Table 3.
The impact of selenium nanoparticles on the growth properties of broiler chickens.
| Groups | Performance traits (g) |
||||
|---|---|---|---|---|---|
| IBW | FBW | BWG | FI | FCR | |
| T1 | 45.00 ± 0.0 | 1960 ± 2.1b | 1915.00 ± 2.0b | 3200 ± 1.1b | 1.710 ± 1.1b |
| T2 | 45.36 ± 0.1 | 2180 ± 1.9a | 2134.64 ± 3.0a | 3150 ± 1.3bc | 1.550 ± 0.9c |
| T3 | 45.10 ± 0.3 | 1860 ± 3.2c | 1814.91 ± 1.5d | 3550 ± 1.8a | 1.840 ± 0.7a |
| T4 | 45.22 ± 0.2 | 1901 ± 2.1bc | 1855.78 ± 0.9c | 3050 ± 1.5c | 1.480 ± 0.6d |
| T5 | 45.00 ± 0.0 | 1900 ± 1.1bc | 1855.12 ± 1.1c | 3080 ± 2.0c | 1.550 ± 1.3c |
The values are presented as mean ± SE, lowercase letters (a–d) in each row indicate the significant differences between different treatments on the growth properties of broiler chickens using LSD test at P < 0.05. T1: Non infected chicks; T2: Non infected chicks + SeNPs 500 mg/kg diet for 35 d; T3: E. tenella-infected chicks and non-treated; T4: E. tenella-infected chicks + treated with SeNPs 500 mg/kg; diet for 5 d; T5: E. tenella-infected chicks + treated with sulfadimidine, 16% solution 8 mL/L of drinking water for 5 d.
Carcass Traits
Table 4 shows a medium significant difference (P = 0.045) between chicks' groups in carcass weight. The lowest carcass weight was detected in T3 infected with E. tenella (75.9 mg), while the highest was in T2 (78.3 mg). Also, the other organs (liver, spleen, and heart) were maintained with SeNPs treatment; the highest weights (1.79, 0.085, and 0.56 mg) detected in SeNPs-treated groups when compared to infected and noninfected control excluding spleen showed the highest weight in T3 may be contributed to Eimeria infection. The fat content was decreased in SeNPs, either infected or noninfected, recording the lowest values (1.20 and 1.18 mg) followed by T5; however, the breast weight was increased by SeNPs treatments with an 18 to 20% increase above controls.
Table 4.
The impact of selenium nanoparticles on the carcass properties of broiler chickens.
| Groups | Carcass (mg) | Liver (mg) | Spleen (g) | Heart (g) | Fat (g) | Breast muscle (g) |
|---|---|---|---|---|---|---|
| T1 | 77.2 ± 0.0ab | 1.78 ± 0.1ab | 0.081 ± 0.01c | 0.55 ± 0.02ab | 1.35 ± 0.1a | 33.11 ± 0.2c |
| T2 | 78.3 ± 0.2a | 1.79 ± 0.2a | 0.085 ± 0.02b | 0.57 ± 0.05a | 1.20 ± 0.2cd | 39.65 ± 0.3a |
| T3 | 75.9 ± 0.2c | 1.71 ± 0.2c | 0.089 ± 0.01a | 0.50 ± 0.03d | 1.30 ± 0.3b | 32.40 ± 0.5d |
| T4 | 76.9 ± 0.6b | 1.76 ± 0.3b | 0.079 ± 0.03d | 0.54 ± 0.04c | 1.18 ± 0.6d | 37.89 ± 0.8b |
| T5 | 76.2 ± 0.3b | 1.75 ± 0.2bc | 0.078 ± 0.06d | 0.52 ± 0.00cd | 1.23 ± 0.3c | 37.12 ± 0.2b |
The values are presented as mean ± SE, lowercase letters (a–d) in each row indicate the significant differences between different treatments on the carcass properties of broiler chickens using LSD test at P < 0.05. T1: Non infected chicks; T2: Non infected chicks + SeNPs 500 mg/kg diet for 35 d; T3: E. tenella-infected chicks and non-treated; T4: E. tenella-infected chicks + treated with SeNPs 500 mg/kg; diet for 5 d; T5: E. tenella-infected chicks + treated with sulfadimidine, 16% solution 8 mL/L of drinking water for 5 d.
Gut Microbiota
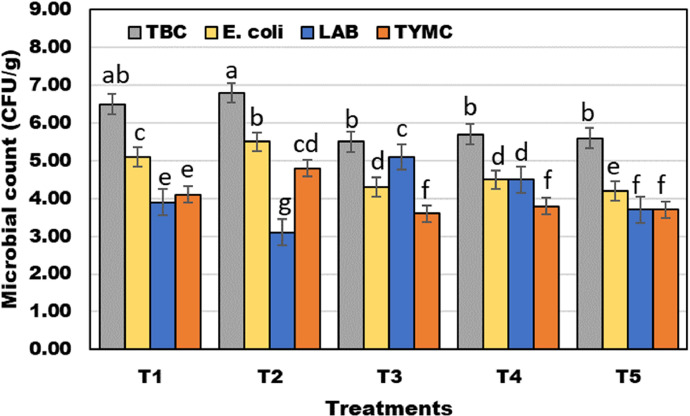
Figure 7 shows the gut microorganism's count. Total bacterial count, yeasts and molds, and E. coli show the highest values with the control and Eimeria-infected brolier group coefficient of 8.5, 4.9, and 7.76 Log10 CFU/g, respectively. It decreases with the augment in the dietary SeNPs level until it reaches the lowest significant value with the treatment SeNPs 500 µg/kg (6.6, 3.7, and 6.57 Log 10 CFU/g). As for lactic acid bacteria count, the lowest value appears with the control and Eimeria-infected brolier group 4 and 3.2, respectively; the values increase with the addition of bacterial SeNPs 500 µg/kg in the diet to achieve the peak height with treatment SeNPs 500 µg/kg (5.2 Log 10 CFU/g), consequently reduced the Eimeria count.
Figure 7.
Effect of SeNPs on the gut microbiota in the cecum of E. tenella-infected broiler. The values are presented as mean ± SE. Lowercase letters (a–g) above columns indicate the significant differences between different treatments on the gut microbiota using the LSD test at P < 0.05. Group 1 (T1) control, Group 2 (T2) E. tenella-infected chicks, Group 3 (T3) chicks supplemented with SeNPs (500 mg/kg diet) for 35 d. Group 4 (T4), E. tenella-infected chicks and then treated with SeNPs (500 mg/kg diet) based on in vitro study. Group 5 (T5), E. tenella-infected chicks and then treated with the anticoccidial agent (sulfadimidine, 16% solution 8 mL/L of drinking water).
Biochemistry and Hematological Parameters
Table 5 shows Eimeria-infected broiler induced the ALT and AST enzymes (28.3 and 81.2) with a relative increase of 50 and 20% compared to the control. The liver enzymes were downregulated to normal levels by SeNPs (500 µg/kg diet) addition in the diet because they mitigate the Eimeria infection. Similarly, the activity of stress content (MDA and NO) increased by infection compared to control, while SeNPs controlled that. On the other hand, the antioxidant enzymes (GPx, GSH, and SOD) decreased by 50% in T3 (Eimeria infected) while supplementing the broiler diet with SeNPs upregulated the activity of these enzymes to normal levels, that is, 60, 22, and 4.6 with no significant difference compared to control.
Table 5.
Effect of SeNPs on the biochemistry and hematology of broiler chickens.
| Blood traits | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| Liver enzymes | |||||
| ALT | 17.1 ± 0.2c | 9.3 ± 0.6d | 28.3 ± 0.6a | 17.9 ± 0.3c | 20.3 ± 0.6b |
| AST | 59.2 ± 0.3c | 38.5 ± 0.1d | 81.2 ± 0.3a | 60.2 ± 0.1c | 68.1 ± 0.5b |
| Antioxidant enzymes | |||||
| GSH | 62.1 ± 0.2a | 60.2 ± 0.3b | 33.6 ± 0.9d | 60.9 ± 0.1b | 57.5 ± 0.2c |
| GPx | 29.8 ± 0.1b | 32.1 ± 0.5a | 11.3 ± 0.8d | 22.6 ± 0.5c | 21.1 ± 0.1c |
| SOD | 4.6 ± 0.2b | 4.8 ± 0.1a | 2.9 ± 0.4d | 4.6 ± 0.4b | 4.1 ± 0.6c |
| Stress components | |||||
| MDA | 18.7 ± 0.2d | 19.2 ± 0.3d | 45.9 ± 0.2a | 21.6 ± 0.6c | 25.7 ± 0.6b |
| NO | 8.9 ± 0.1d | 9.1 ± 0.1d | 22.5 ± 0.5a | 10.22 ± 0.1c | 12.1 ± 0.8b |
| Serum biochemical | |||||
| Protein (g/dL) | 2.70 ± 0.1b | 2.9 ± 0.9a | 1.0 ± 0.0e | 2.5 ± 0.1c | 2.0 ± 0.1d |
| Albumin (g/dL) | 1.36 ± 0.2b | 1.5 ± 0.1a | 1.1 ± 0.01d | 1.41 ± 0.2ab | 1.32 ± 0.2c |
| Globulin (g/dL) | 1.30 ± 0.6c | 1.43 ± 0.5a | 0.95 ± 0.02d | 1.39 ± 0.6b | 1.31 ± 0.3c |
| A/G ratio | 1.07 ± 0.5b | 1.04 ± 0.7b | 1.15 ± 0.01a | 1.01 ± 0.4c | 1.00 ± 0.1c |
| Lipids (mg/dL) | 0.42 ± 0.4b | 0.35 ± 0.5c | 0.58 ± 0.05a | 0.31 ± 0.06d | 0.34 ± 0.04c |
| Hematological parameters | |||||
| RBCs (106/µL) | 2.4 ± 0.2bc | 2.7 ± 0.3a | 1.9 ± 0.2d | 2.5 ± 0.1b | 2.3 ± 0.2c |
| WBCs 102/µL) | 138 ± 0.8b | 130 ± 1.1c | 145 ± 1.2a | 131 ± 1.2c | 136 ± 1.2bc |
| PLT (104/µL) | 39.2 ± 0.8b | 32.6 ± 0.6d | 48.1 ± 0.2a | 33.6 ± 0.6d | 36.6 ± 0.2c |
The values are presented as mean ± SE, lowercase letters (a–e) in each row indicate the significant differences between different treatments on the biochemistry and hematology of broiler chickens using LSD test at P < 0.05. T1: Non infected chicks; T2: Non infected chicks + SeNPs 500 mg/kg diet for 35 d; T3: E. tenella-infected chicks and non-treated; T4: E. tenella-infected chicks + treated with SeNPs 500 mg/kg; diet for 5 d; T5: E. tenella-infected chicks + treated with sulfadimidine, 16% solution 8 mL/L of drinking water for 5 d.
Concerning the biochemistry of broiler blood, albumin and globulin contents were decreased by 30% in E. tenella-infected chicks compared with normal control; the addition of SeNPs increased the protein content by 1.5-fold than T3, while T4 and T5 recorded the lowest values in lipid profile. During Eimeria infection, the WBCs and PLT counts significantly increased while RBCs decreased while SeNPs corrected this content to control levels.
DISCUSSION
Eimeria infection symptoms include malnutrition, enteritis, and, in severe cases, mortality for some Eimeria species, endangering the health of birds and their economic production (Youssefi et al., 2023). Food security and the global agroeconomy will face significant challenges due to this pathogenic protozoan and its detrimental impact on production due to the ever-increasing human need for protein resources from poultry (Teng et al., 2023). Since the 1940s, over 30 sulfanilamide medications have been used to treat and control coccidiosis (Ekinci et al., 2023). These anticoccidial treatments affect Eimeria species' cofactor production, mitochondrial activity, or cell membrane function (Peek, 2010). Commercial pharmaceuticals need to develop novel anti-Eimeria therapies because of concerns about health and the environment besides parasite resistance (Wunderlich et al., 2014). In this study, we used sulfadimidine as an anticoccidial reference, and many studies have proven its efficiency (Rabo et al., 2021). This study used experimentally infected cockerels to determine the efficacy of amprolium, septrin, and sulfadimidine in controlling Eimeria necatrix infection. The infection was characterized by weight loss, anemia, dullness, bloody diarrhea, and death. Of the 3 drugs, septrin was found to be most effective in treating the disease, followed by sulfadimidine and amprolium, as evidenced by the amelioration of clinical signs, gross and histopathological lesions, and relative survival rates of treated birds. Also, Kuraa et al. (2021)confirmed that in vitro sporulation inhibition of garlic extract showed significant efficacy on E. magna oocysts in comparison with black seed extract and high significant efficacy of sporulation inhibition, compared to sulfadimidine and it referred to that sulfadimidine has both in vitro and in vivo an inhibitory effect against sporulation.
An experimental study was conducted to assess the efficacy of amprolium and sulfadimidine drugs in treating chickens’ coccidiosis. A total of 52 chickens were purposively selected for this study. Among them, 17 chickens were used for the control group, while 35 were used for the treatment group. Of the 35 chickens used for the treatment group, 18 were treated with amprolium, and the remaining 17 were treated with sulfadimidine. The drugs were administered orally in drinking water.
The treatment response of these drugs for coccidiosis concerning the reduction in fecal scores (OPG count); the study showed that there was no statistically significant difference in OPG count between before and after treatment in amprolium-treated groups (P > 0.05), but in the sulfadimidine administered group, there was a statistically significant difference (P = 0.004). This reveals that sulfadimidine is more effective than amprolium in treating coccidial infection (Hunduma and Kebede, 2016). Furthermore, in the study of Khan et al. (2021) they found that sulfadimidine was most effective (45%) followed by amprolium (44.6%), while triquen (24.0%) showed less effectiveness against coccidiosis in pigeons.
Nanoparticles enable the development of innovative antiparasitic medicinal medicines (Alkhudhayri et al., 2020). For example, nanoselenium (SeNPs) has been used to treat different parasites (Dkhil et al., 2016; Alkhudhayri et al., 2020; Malekifard et al., 2020).
In addition, selenium at the nanoscale provides several health advantages as SeNPs possess antioxidant and anti-inflammatory effects, according to Rayman (2008). Also, antimicrobial, antiviral, and antiparasitic activities have been established (Abou Elmaaty et al., 2022, Salem et al., 2022a). SeNPs also showed anticoccidial properties (Alkhudhayri et al., 2018). This work analyses the possible anticoccidial activity of SeNPs derived from P. polymyxa, which is likely attributable to the composition of the bacterial pellets and the unique features of SeNPs.
Bio-SeNPs effectively lowered the number of oocysts shed by chicks by about 97.21% on the 10th day following infection. Most anticoccidial treatments are known to decrease intracellular Eimeria stages and reduce the oocyst shedding rate effect; some studies confirmed our results on the effect of SeNPs on Eimeria but were conducted on mice (Alkhudhayri et al., 2020), they found that the numbers of meronts, gamonts, and developing oocysts of E. papillata reduced after the infected mice were treated with the SeNPs.
Anticoccidial activities may be attributable to the high quantities of active components detected in bacterial supernatants (Oke et al., 2020). These chemicals have a significant antibacterial action by competing with the bacterial membranes, affecting their porosity for water and cations, leading to diminished function, cellular component leakage, and cell demise (Filipović et al., 2021).
Infection with E. tenella is followed by the inflammation of cecum cells and oxidative damage in chickens; while permitting an increase in the total count of bacteria, E. coli, total yeast, and molds, and reducing the lactic acid bacteria, these effects were reversed when added dietary selenium agreeing with Al-Quwaie (2023), who found that the addition of selenium nanoparticles (BSeNPs) and elemental selenium (Se) significantly reduced the levels of total bacterial count (TBC), total yeast mold count (TYMC), E. coli, and Salmonella in comparison to the control group. However, the levels of lactic acid bacteria (LAB) were significantly increased.
The treatment of infected animals with SeNPs significantly mitigated the oxidative injury of the cecal tissue. The powerful potential impact of SeNPs is attributable to their distinct properties, such as the antioxidant anti-inflammation activity of SeNPs and stabilizing agent (Nabi et al., 2020; Qudoos et al., 2020; Abdel-Moneim et al., 2022).
The coccidial infection disturbs the work of the antioxidant defense system against free radical generation (Murshed et al., 2023). Our results demonstrated that infection with E. tenella is linked to oxidative stress in broiler chickens' cecum, reducing the levels of GSH, GPx, and SOD enzymes, and these antioxidant defense systems are essential for preventing free radical damage to the animal body during an Eimeria infection. SeNPs increase these enzymes' activity, which is generally lowered during infection-induced oxidative damage (Chen et al., 2021).
Lipid oxidation leads to the formation of several carbonyl compounds, including MDA (Michalak et al., 2022). Following The SeNPs treatment, the MDA levels drop. The NO levels rose in response to pathogenic sporozoite phases that penetrated and inflamed cecal cells. In addition, the imbalance caused an infection-related rise in nitric oxide levels (Dominguez et al., 2015). Bio-SeNPs might significantly lessen the incidence of cecal infection by boosting NO levels. This demonstrates the antioxidant potential of SeNPs. According to previous research, SeNPs and plant extracts include necessary active chemicals (Seriana et al., 2021). Bacterial SeNPs and the stabilizing agent may indicate their biological activity in this work.
This study indicated how E. tenella infection reduced the expression of the MUC2 gene, as per Ciszewski et al. (2022).
A significant increase in IL-6 and TNF-α was indicative of the inflammation response to the E. tenella infection. Mucin synthesis is controlled by proinflammatory cytokines such as IL-6 and TNF-α (Parrish et al., 2022). In contrast to the current work, Eimeria parasite infection-induced cytokine influx and mucin glycosylation downregulation. These adverse effects are linked to the reduction of goblet cells and mucus thickness and accelerated transport of mucin from the Golgi to the secretory vesicles (Liu et al., 2022). The SeNPs treatment significantly decreased the inflammation response in infected chicks.
Additionally, to face the Eimeria stages within infected tissues, our research indicated that SeNPs act as an anti-inflammation to protect host tissues. This anti-inflammatory action may be caused by active compounds in SeNPs and their precursors (Abdel-Moneim et al., 2022). In addition to immunomodulatory and anti-inflammatory properties, it can prevent the production of proinflammatory cytokines (Attia et al., 2020, Nagata et al., 2023), according to previous studies. Apoptosis may influence the chicks' response to particular intracellular parasite infections and promote the removal of damaged or contaminated cells (Al-Quraishy et al., 2019). The high levels of proapoptotic genes BCL2 and caspase-3 indicate the damage to cecum cells. Previous research revealed increased apoptotic cells in the jejunum of E. papilloma-infected mice (Dkhil et al., 2016). Bio-SeNPs may ameliorate the meiosis-induced apoptotic changes in jejunal cells, as reported by many studies (Chen et al., 2022).
According to the study, SeNPs enhanced the development performance of chickens. Nano-Se improved the growth performance of treated hens, suggesting that it is more bioavailable and less harmful than inorganic forms of Se (Ibrahim et al., 2019). Our results contradict those of Yoon et al. (2007), who indicated that the Se form in the chicks' food did not affect their development performance. In contrast, the performance of chicks supplemented with 200 g/kg of organic Se or nano-Se was comparable to that of chicks given the same quantity of Se as selenite (Couloigner et al., 2015). Selenium is required for the production of hormones and also to enhance the healthy development of avian creatures (Wang et al., 2011) and has been shown to affect bird growth performance.
Using SeNPs in this study improved bird antioxidant status. Also, Visha et al. (2017) observed comparable results in birds fed nano- and organic Se; their blood and tissues showed better antioxidant capacity than those fed inorganic selenium by modulating the scavenging abilities of seleno-enzymes. Se plays a crucial role in protecting cells from oxidative damage (Sarkar et al., 2011).
In addition to serum oxidants and selenium retention in vivo, nano-Se can neutralize free radicals by boosting the activity of selenium-based enzymes and promoting growth (Sarkar et al., 2011). Unlike other selenium forms, nano-Se exhibited decreased acute toxicity but elevated selenoenzyme activity. GPXs and thioredoxin reductase are mostly responsible for the antioxidant activity of nano-Se (TR). Multiple peroxides, including H2O2, phospholipid hydroperoxide, fatty acid hydroperoxides, and thymine hydroperoxyl groups, can be eliminated by the GPXs (Kondaparthi et al., 2019). The study's outcome was that the inclusion of nano-Se in chickens' food can effectively increase their antioxidant potential, consequently enhancing their ability to detoxify a wide variety of peroxides, improving bird antioxidant status, performance, and blood parameters and reducing the negative impact of E. tenella experimental infection.
CONCLUSIONS
Remarkably, a 500 µg/kg diet of bacteriogenic SeNPs revealed both in vitro and in vivo anticoccidial action against E. tenella. The dietary supplementation of 500 µg/kg bacteriogenic SeNPs improved growth performance, gut health, carcass traits, biochemistry, and hematological indices and exhibited significant antioxidant activity in the treated chickens.
Acknowledgments
Acknowledgments
This work was funded by the University of Jeddah, Jeddah, Saudi Arabia, Under grant No. (UJ-22-DR-87). The authors therefore, acknowledge with thanks the University of Jeddah for its technical and financial support.
Author Contributions
Conceptualization, M. N. A., and M. T. E.-S., formal Analysis, M. N. A., and M. T. E.-S., investigation, M. N. A., and M. T. E.-S., data curation, M. N. A., and M. T. E.-S., writing original draft preparation, M. N. A., and M. T. E.-S., writing final manuscript and editing, M. N. A., and M. T. E.-S., visualization and Methodology, M. N. A., and M. T. E.-S. All authors have read and agreed to the published version of the manuscript.
DISCLOSURES
The author declares that they have no conflicts of interest.
REFERENCES
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., Nahed A., Saad A.M., Salem H.M., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives – a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.E., El-Saadony M.T., Shehata A.M., Saad A.M., Aldhumri S.A., Ouda S.M., Mesalam N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022;29:1197–1209. doi: 10.1016/j.sjbs.2021.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Elmaaty T., Sayed-Ahmed K., Elsisi H., Ramadan S.M., Sorour H., Magdi M., Abdeldayem S.A. Novel antiviral and antibacterial durable polyester fabrics printed with selenium nanoparticles (SeNPs) Polymers. 2022;14:955. doi: 10.3390/polym14050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam H., Caihak G. Fischer Verlag; Stuttgart, Germany: 1964. Grosses zoologisches parktikum tell. Arbeitsmethoden der makroskopischen und mikroskopischen anatomic Mit 283 Abbildungen Gustav. [Google Scholar]
- Alagawany M., El-Saadony M.T., El-Rayes T.K., Madkour M., Loschi A.R., Di Cerbo A., Reda F.M. Evaluation of dried tomato pomace as a non-conventional feed: its effect on growth, nutrients digestibility, digestive enzyme, blood chemistry and intestinal microbiota of growing quails. Food Energy Secur. 2022;11:e373. [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Alkhudhayri A., Al-Shaebi E.M., Qasem M.A., Murshed M., Mares M.M., Al-Quraishy S., Dkhil M.A. Antioxidant and anti-apoptotic effects of selenium nanoparticles against murine eimeriosis. Ann. Acad. Bras. Cienc. 2020;92:1–9. doi: 10.1590/0001-3765202020191107. [DOI] [PubMed] [Google Scholar]
- Alkhudhayri A.A., Dkhil M.A., Al-Quraishy S. Nanoselenium prevents eimeriosis-induced inflammation and regulates mucin gene expression in mice jejunum. Int. J. Nanomed. 2018;13:1993–2003. doi: 10.2147/IJN.S162355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alowaiesh B.F., Alhaithloul H.A.S., Saad A.M., Hassanin A.A. Green biogenic of silver nanoparticles using polyphenolic extract of olive leaf wastes with focus on their anticancer and antimicrobial activities. Plants. 2023;12:1410. doi: 10.3390/plants12061410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Quraishy S., Thagfan F.A., Al-Shaebi E.M., Qasem M., Abdel-Gaber R., Dkhil M.A.M. Salvadora persica protects mouse intestine from eimeriosis. Rev. Bras. DE Parasitol. Vet. 2019;28:605–612. doi: 10.1590/S1984-29612019068. [DOI] [PubMed] [Google Scholar]
- Al-Quwaie D.A. The influence of bacterial selenium nanoparticles biosynthesized by Bacillus subtilus DA20 on blood constituents, growth performance, carcass traits, and gut microbiota of broiler chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi T., Wami E. Biocorrosion of mild steel in culture of aerobic bacteria. J. Newviews Eng. Technol. 2023;5:12–21. [Google Scholar]
- Arafa F.M., Mogahed N.M., Eltarahony M.M., Diab R.G. Biogenic selenium nanoparticles: trace element with promising anti-toxoplasma effect. Pathog. Glob. Health. 2023;9:1–16. doi: 10.1080/20477724.2023.2186079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., El-Gameel S.M., Ismael E. Evaluation of tumor necrosis factor-alpha (TNF-α); gamma interferon (IFN-γ) genes and oxidative stress in sheep: immunological responses induced by Oestrus ovis (Diptera: Oestridae) infestation. J. Parasit. Dis. 2020;44:332–337. doi: 10.1007/s12639-020-01220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M.M., Yehia N., Soliman M.M., Shukry M., El-Saadony M.T., Salem H.M. Evaluation of the antiparasitic activity of the chitosan-silver nanocomposites in the treatment of experimentally infested pigeons with Pseudolynchia canariensis. Saudi J. Biol. Sci. 2022;29:1644–1652. doi: 10.1016/j.sjbs.2021.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht N., Phalswal P., Khanna P.K. Selenium nanoparticles: a review on synthesis and biomedical applications. Mater. Adv. 2022;3:1415–1431. [Google Scholar]
- Carvalho G.C., Sábio R.M., de Cássia Ribeiro T., Monteiro A.S., Pereira D.V., Ribeiro S.J.L., Chorilli M. Highlights in mesoporous silica nanoparticles as a multifunctional controlled drug delivery nanoplatform for infectious diseases treatment. Pharm. Res. 2020;37:191–221. doi: 10.1007/s11095-020-02917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.D. Applied strategies for the control of coccidiosis in poultry. CABI Rev. 2018;13:1–11. [Google Scholar]
- Chen G., Yang F., Fan S., Jin H., Liao K., Li X., Liu G.-B., Liang J., Zhang J., Xu J.-F. Immunomodulatory roles of selenium nanoparticles: novel arts for potential immunotherapy strategy development. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.956181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Qiao L., Song X., Ma L., Dou X., Xu C. Protective effects of selenium nanoparticle-enriched Lactococcus lactis NZ9000 against enterotoxigenic Escherichia coli K88-induced intestinal barrier damage in mice. Appl. Environ. Microbiol. 2021;87 doi: 10.1128/AEM.01636-21. e01636-01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciszewski A., Jarosz Ł.S., Kalinowski M., Marek A., Grądzki Z., Grabowski S., Hejdysz M., Nowaczewski S., Rysiak A. Influence of effective microorganisms and clinoptilolite on gut barrier function, intestinal health and performance of broiler chickens during induced Eimeria tenella infection. Agriculture. 2022;12:2176. [Google Scholar]
- Couloigner F., Jlali M., Briens M., Rouffineau F., Geraert P.-A., Mercier Y. Selenium deposition kinetics of different selenium sources in muscle and feathers of broilers. Poult. Sci. 2015;94:2708–2714. doi: 10.3382/ps/pev282. [DOI] [PubMed] [Google Scholar]
- Davies S.F.M., Joyner L.P., Kendall S.B. Oliver & Boyd; Edinburgh and London, UK: 1963. Coccidiosis. [Google Scholar]
- Dkhil M.A., Al-Quraishy S., Abdel Moneim A.E., Delic D. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol. Res. 2013;112:101–106. doi: 10.1007/s00436-012-3109-1. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A., Bauomy A., Diab M.S., Al-Quraishy S. Protective role of selenium nanoparticles against Schistosoma mansoni induced hepatic injury in mice. Biomed. Res. (India) 2016;27:214–219. [Google Scholar]
- Dominguez P., Pro-Martinez A., Narciso-Gaytán C., Hernández-Cázares A., Sosa-Montes E., Perez-Hernandez P., Caldwell D., Ruiz-Feria C. Concurrent supplementation of arginine and antioxidant vitamins E and C reduces oxidative stress in broiler chickens after a challenge with Eimeria spp. Can. J. Anim. Sci. 2015;95:143–153. [Google Scholar]
- Ekinci İ.B., Chłodowska A., Olejnik M. Ionophore toxicity in animals: a review of clinical and molecular aspects. Int. J. Mol. Sci. 2023;24:1696. doi: 10.3390/ijms24021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ashry R.M., El-Saadony M.T., El-Sobki A.E., El-Tahan A.M., Al-Otaibi S., El-Shehawi A.M., Saad A.M., Elshaer N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 2022;29:920–932. doi: 10.1016/j.sjbs.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Maddawy Z.K., El-Sawy A.E.-S.F., Ashoura N.R., Aboelenin S.M., Soliman M.M., Ellakany H.F., Elbestawy A.R., El-Shall N.A. Use of zinc oxide nanoparticles as anticoccidial agents in broiler chickens along with its impact on growth performance, antioxidant status, and hematobiochemical profile. Life. 2022;12:74. doi: 10.3390/life12010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah H.I.A., Mahgoub S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7:238–249. [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Selem E., Desoky E.-S.M., Fouda S.E., El-Tahan A.M. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021;28:4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28:6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipović N., Ušjak D., Milenković M.T., Zheng K., Liverani L., Boccaccini A.R., Stevanović M.M. Comparative study of the antimicrobial activity of selenium nanoparticles with different surface chemistry and structure. Front. Bioeng. Biotechnol. 2021;8 doi: 10.3389/fbioe.2020.624621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Bellet C., Blake D.P., Tomley F.M., Rushton J. Revisiting the economic impacts of Eimeria and its control in European intensive broiler systems with a recursive modeling approach. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.558182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi H., Firouzi S., Nili H., Razavi M., Asadi S.L., Daneshi S. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: in vitro and in vivo study. J. Parasit. Dis. 2016;40:401–407. doi: 10.1007/s12639-014-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H.-W., Patel KD., Kwak J.-H., Jun S.-K., Jang T.-S., Lee S.-H., Knowles J.C., Kim H.-W., Lee H.-H., Lee J.-H. Selenium nanoparticles as candidates for antibacterial substitutes and supplements against multidrug-resistant bacteria. Biomolecules. 2021;11:1028. doi: 10.3390/biom11071028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunduma A., Kebede B. Comparative study on the efficacy of amprolium and sulfadimidine in coccidia infected chickens in Debre-Zeit agricultural research center poultry farm, Bishoftu, Ethiopia. SOJ Vet. Sci. 2016;2:1–5. [Google Scholar]
- Ibrahim D., Kishawy A.T., Khater S.I., Hamed Arisha A., Mohammed H.A., Abdelaziz A.S., Abd El-Rahman G.I., Elabbasy M.T. Effect of dietary modulation of selenium form and level on performance, tissue retention, quality of frozen stored meat and gene expression of antioxidant status in ross broiler chickens. Animals. 2019;9:342. doi: 10.3390/ani9060342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W., Das S.N., Mahmoud A.H., Rafique N., Anwar K., Khan B.T., Mohammed O.B. Evaluation of sulfadimidine, amprolium and triquen to treat coccidiosis in wild pigeons. Braz. J. Biol. 2021;82 doi: 10.1590/1519-6984.238673. [DOI] [PubMed] [Google Scholar]
- Kondaparthi P., Flora S., Naqvi S. Selenium nanoparticles: an insight on its Pro-oxidant and antioxidant properties. Front. Nanosci. Nanotechnol. 2019;6:1–5. [Google Scholar]
- Kuraa H.M.M., Nageib B.R., El-Hendy A.H.M., Hassanin A.A.A. Evaluation of prophylactic and anticoccidial effects of black seed and garlic extracts in rabbits. World Vet. J. 2021;11:124–137. [Google Scholar]
- Liang T.-W., Wang S.-L. Recent advances in exopolysaccharides from Paenibacillus spp.: production, isolation, structure, and bioactivities. Mar. Drugs. 2015;13:1847–1863. doi: 10.3390/md13041847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Niu X., Li Y., Zhang J.-r., Zhu S.-j., Yang Q.-y., Zhang W., Gong L. Role of the mucin-like glycoprotein FCGBP in mucosal immunity and cancer. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.863317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang C., Li Y., Liu K., Hou Q., Xu W., Fan L., Zhao J., Gou J., Du B. Complete genome sequence of Paenibacillus polymyxa YC0573, a plant growth–promoting rhizobacterium with antimicrobial activity. Genome Announc. 2017;5 doi: 10.1128/genomeA.01636-16. e01636-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Malekifard F., Tavassoli M., Vaziri K. In vitro assessment antiparasitic effect of selenium and copper nanoparticles on Giardia deodenalis cyst. Iran. J. Parasitol. 2020;15:411. doi: 10.18502/ijpa.v15i3.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak I., Dziergowska K., Alagawany M., Farag M.R., El-Shall N.A., Tuli H.S., Emran T.B., Dhama K. The effect of metal-containing nanoparticles on the health, performance and production of livestock animals and poultry. Vet. Quart. 2022;42:68–94. doi: 10.1080/01652176.2022.2073399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshed M., Al-Tamimi J., Aljawdah H.M.A., Al-Quraishy S. Pharmacological effects of grape leaf extract reduce eimeriosis-induced inflammation, oxidative status change, and goblet cell response in the Jejunum of mice. Pharmaceuticals. 2023;16:928. doi: 10.3390/ph16070928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi F., Arain M., Hassan F., Umar M., Rajput N., Alagawany M., Syed S., Soomro J., Somroo F., Liu J. Nutraceutical role of selenium nanoparticles in poultry nutrition: a review. World's Poult. Sci. J. 2020;76:459–471. [Google Scholar]
- Nagata K., Araumi S., Ando D., Ito N., Ando M., Ikeda Y., Takahashi M., Noguchi S., Yasuda Y., Nakano N. Kaempferol suppresses the activation of mast cells by modulating the expression of FcεRI and SHIP1. Int. J. Mol. Sci. 2023;24:5997. doi: 10.3390/ijms24065997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack S., Chapman H.D., Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019;118:2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke E., Adeyi O., Okolo B., Adeyi J., Ayanyemi J., Osoh K., Adegoke T. Phenolic compound extraction from Nigerian Azadirachta indica leaves: response surface and neuro-fuzzy modelling performance evaluation with Cuckoo search multi-objective optimization. Results Eng. 2020;8 [Google Scholar]
- Parrish A., Boudaud M., Kuehn A., Ollert M., Desai M.S. Intestinal mucus barrier: a missing piece of the puzzle in food allergy. Trends Mol. Med. 2022;28:36–50. doi: 10.1016/j.molmed.2021.10.004. [DOI] [PubMed] [Google Scholar]
- Peek, H. 2010. Resistance to anticoccidial drugs: alternative strategies to control coccidiosis in broilers. Doctoral Diss. Utrecht University, Utrecht.
- Prokisch, J., M. A. Zommara. 2011. Process for producing elemental selenium nanospheres. Google Patents.
- Qudoos A., Iqbal A., Ahmad S.S., Khan M.S., Bayram İ. Effects of some alternative plant extracts used as natural coccidiostat for pigeons. J. Anim. Sci. Prod. 2020;3:20–31. [Google Scholar]
- Rabo J.S., Biu A.A., Casimir N.K. Experimental Eimeria necatrix infection: comparative efficacy of sulphadimidine, amprolium and spectrin in cockerels. Biomed. Res. J. 2021;14:51–55. [Google Scholar]
- Rayman M.P. Food-chain selenium and human health: emphasis on intake. Br. J. Nutr. 2008;100:254–268. doi: 10.1017/S0007114508939830. [DOI] [PubMed] [Google Scholar]
- Rybakova D., Cernava T., Köberl M., Liebminger S., Etemadi M., Berg G. Endophytes-assisted biocontrol: novel insights in ecology and the mode of action of Paenibacillus. Plant Soil. 2016;405:125–140. [Google Scholar]
- Saad A.M., Sitohy MZ., Sultan-Alolama M.I., El-Tarabily K.A., El-Saadony M.T. Green nanotechnology for controlling bacterial load and heavy metal accumulation in Nile tilapia fish using biological selenium nanoparticles biosynthesized by Bacillus subtilis AS12. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1015613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem S.S., Badawy M.S.E., Al-Askar A.A., Arishi A.A., Elkady F.M., Hashem A.H. Green biosynthesis of selenium nanoparticles using orange peel waste: characterization, antibacterial and antibiofilm activities against multidrug-resistant bacteria. Life. 2022;12:893. doi: 10.3390/life12060893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Salem M.A., Soliman M.M., Althobaiti S.A., Khafaga A.K., El-Tahan A.M., El-Saadony M.T., Attia M.M. Parasitological and histopathological examination of Cocktail lovebirds infected with Eimeria aratinga (Apicomplexa: Eimeriidae) Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan M., Farghaly A., Abd El-Aal N., Farag S.M., Ali A.A., Farag T. Egyptian propolis and selenium nanoparticles against murine trichinosis: a novel therapeutic insight. J. Helminthol. 2022;96:e50. doi: 10.1017/S0022149X22000359. [DOI] [PubMed] [Google Scholar]
- Sarkar J., Dey P., Saha S., Acharya K. Mycosynthesis of selenium nanoparticles. Micro Nano Lett. 2011;6:599–602. [Google Scholar]
- Seriana I., Akmal M., Darusman D., Wahyuni S., Khairan K., Sugito S. Neem leaf (Azadirachta indica A. Juss) ethanolic extract on the liver and kidney function of rats. Sci. World J. 2021;2021:1–7. doi: 10.1155/2021/7970424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swelum A.A., Elbestawy A.R., El-Saadony M.T., Hussein E.O., Alhotan R., Suliman G.M., Abd El-Hack M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: an updated overview. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.Y., Liu G., Choi J., Yadav S., Wei F., Kim W.K. Effects of levels of methionine supplementations in forms of L-or DL-methionine on the performance, intestinal development, immune response, and antioxidant system in broilers challenged with Eimeria spp. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thienpont D., Rochette F., Vanparijs O.F.J. Book Review: Diagnosing Helminthiasis Through Coprological Examination (ED. 2) Turnhoutseweg; Beerse, Belgium: 1986. [Google Scholar]
- Visha P., Nanjappan K., Selvaraj P., Jayachandran S., Thavasiappan V. Influence of dietary nanoselenium supplementation on the meat characteristics of broiler chickens. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:340–347. [Google Scholar]
- Wallenstein S., Zucker C.L., Fleiss J.L. Some statistical methods useful in circulation research. Circ. Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhan X., Zhang X., Wu R., Yuan D. Comparison of different forms of dietary selenium supplementation on growth performance, meat quality, selenium deposition, and antioxidant property in broilers. Biol. Trace Elem. Res. 2011;143:261–273. doi: 10.1007/s12011-010-8839-2. [DOI] [PubMed] [Google Scholar]
- Wiedosari E., Wardhana A.H. Anticoccidial activity of artemisinin and extract of Artemesia annua leaves in chicken infected by Eimeria tenella. J. Ilmu. Ternak Dan. Vet. 2018;22:196–204. [Google Scholar]
- Wunderlich F., Al-Quraishy S., Steinbrenner H., Sies H., Dkhil M.A. Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol. Res. 2014;113:3547–3556. doi: 10.1007/s00436-014-4101-8. [DOI] [PubMed] [Google Scholar]
- Yang D., Jacobson A., Meerschaert K.A., Sifakis J.J., Wu M., Chen X., Yang T., Zhou Y., Anekal P.V., Rucker R.A. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell. 2022;185:4190–4205. doi: 10.1016/j.cell.2022.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon I., Werner T., Butler J. Effect of source and concentration of selenium on growth performance and selenium retention in broiler chickens. Poult. Sci. 2007;86:727–730. doi: 10.1093/ps/86.4.727. [DOI] [PubMed] [Google Scholar]
- Yousry C., Zikry P.M., Salem H.M., Basalious E.B., El-Gazayerly O.N. Integrated nanovesicular/selfnanoemulsifying system (INV/SNES) for enhanced dual ocular drug delivery: statistical optimization, in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2020;10:801–814. doi: 10.1007/s13346-020-00716-5. [DOI] [PubMed] [Google Scholar]
- Youssefi M.R., Alipour R., Fakouri Z., Shahavi M.H., Nasrabadi N.T., Tabari M.A., Centoducati G. Dietary supplementation with eugenol nanoemulsion alleviates the negative effects of experimental coccidiosis on broiler chicken's health and growth performance. Molecules. 2023;28:2200. doi: 10.3390/molecules28052200. [DOI] [PMC free article] [PubMed] [Google Scholar]