Abstract
The superb specificity and potency of biological toxins targeting various ion channels and receptors are of major interest for the delivery of therapeutics to distinct cell types and subcellular compartments. Fused with reporter proteins or labelled with fluorophores and nanocomposites, animal toxins and their detoxified variants also offer expanding opportunities for visualisation of a range of molecular processes and functions in preclinical models, as well as clinical studies. This article presents state-of-the-art optical probes derived from neurotoxins targeting ion channels, with discussions of their applications in basic and translational biomedical research. It describes the design and production of probes and reviews their applications with advantages and limitations, with prospects for future improvements. Given the advances in imaging tools and expanding research areas benefiting from the use of optical probes, described here resources should assist the discovery process and facilitate high-precision interrogation and therapeutic interventions.
Keywords: Animal toxins, Ion channels, Fluorescent probes, ICG, Optical imaging, Visualisation, Advanced biomaterials
Introduction
The main objective of biological imaging is uncompromised visualisation of the structure and function of living organisms in their unperturbed environments. In this pursuit, fluorescence markers and reporter proteins combined with precision delivery and spectral multiplexing have been of critical importance. Through fluorescence effects, optical imaging provides not only insights into the molecular content and structure of living systems but allows visualisation of dynamic processes in real-time, from macroscopic to subcellular and molecular levels in vitro and in vivo [1–4]. The use of fluorescence proteins and probes with specificity for various cellular and molecular interactions has greatly enhanced the imaging of functional processes with their characterisation [5–10]. These advances have been bolstered by endoscopic and hybrid capacities, which enabled capturing multiple features at unprecedented depth, with increasing precision and specificity [11–15].
Improving targeting and delivery of fluorescence probes to various types of cells and subcellular compartments has been an essential part of recent advances in optical imaging [16–21]. Fluor-labelled peptides, functionalized nanocomposites and particles are increasingly considered for a range of preclinical and clinical use, to improve the specificity and efficacy of payload delivery, and to achieve higher imaging sensitivity and contrast, with lower toxicity in single and high throughput studies [22–24]. These developments have pushed the limits of optical imaging to new realms, enabling superb visualising capabilities, from molecular and sub-molecular to system and organism levels [4, 5, 14, 25, 26]. The high specificity and potency of biological toxins owed to a major extent to targeting distinctive sets of molecules are of special interest for precision delivery [27–31]. Since their rise in early living forms, biological toxins have been continuously diversified and refined by selective pressure for higher potency, stability and specificity [32–35]. Due to the critical role of ion channels and neurotransmitter receptors in supporting essential mechanisms and functions of living organisations, they have become favourite targets of biological toxins, including those acting on peripheral and central nervous systems [27, 31, 36, 37].
In pursuit of improving the delivery of fluorescence probes and reporter proteins to a specific cell and tissue type, natural toxins and their detoxified variants have produced considerable interest [30, 31, 38–40]. With a growing number of recombinant forms with lower toxicity, higher specificity and delivery capacity, the expanding portfolio of biological toxins offers an extensive selection of probes and nano-carriers for a range of applications. In this article, we review the state-of-the-art optical probes derived from natural and recombinant animal toxins targeting ion channels and using them for biological and medical imaging (Fig. 1). We discuss reports exploiting neurotoxin-derived probes with specificity for potassium, sodium, calcium, chloride, TRP, acid-sensing and piezo channels, in native and heterologous systems. We conclude our analysis with considerations of key advantages and limitations, with prospects for future improvements of the use of neurotoxin-derived probes in basic and translational research.
Fig. 1.
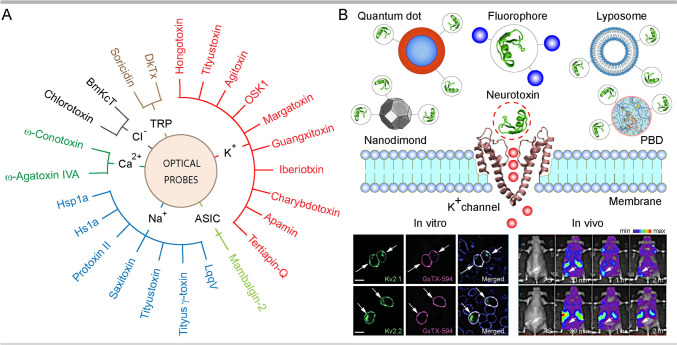
An overview of animal toxins targeting ion channels used for biomedical imaging. A Representation of major neurotoxins with target ion channels utilised for optical imaging in basic and translational studies. B Illustration of general strategy and approaches used for targeting neurotoxin-derived optical probes to ion channels with a representative in vitro (CHO cells expressing KV2.1 and KV2.2 labelled with GxTx labelled with Alexa594) and in vivo (mice grafted with SKOV3 tumours labelled with SOR-C27-Cy5.5) data. Images are reproduced with permission, with detailed explanations provided in respective parts of the review
Optical Probes Targeting Potassium Channels
Potassium channels are one of the largest ion channel families [41, 42]. They are present in many cell types, with expression level and activity altered in a variety of diseases, including several types of cancer [43, 44], neurological and psychiatric conditions [45–48], neurodegenerative diseases [49, 50] and others. Due to their extensive presence in various cell types and biological importance, K+ channels have been a major target of animal toxins, with many exquisitely adapted to bind and interfere with their activity and functions.
Shaker-Related K+ Channels
Shaker channels are the most diverse subfamily of K+ channels, formed by the tetramerization of KV1.1-1.8 subunits [45, 51]. Numerous reports suggest labelled toxins targeting these channels as useful probes for visualising a range of biological processes (Table 1, Fig. 2). Hongotoxin-1 (HgTx1) of scorpion Centruroides limbatus and its recombinant HgTx1-A19C variant derived by site-directed mutagenesis tagged with Cy and Alexa fluorophores were developed and proved highly effective for mapping the distribution of KV1.1 and KV1.2 subunits in basket cell terminals of the rabbit cerebellum [52]. The high affinity of HgTx-A19C to the KV1.3 subunit rendered Cy5-HgTx-A19C also useful for 3D imaging of KV1.3 expression and distribution in Jurkat cells at a single molecule level [53]. The location of channels has been resolved ~40 nm along the x and y axis, with their distribution and dynamics across the cell membrane described. Selective labelling of KV1.3 in Jurkat cells with Cy5-HgTx-A19C and imaging of clustered channels have been confirmed by another report [54]. Using ATTO594-labelled Tityus toxin (TsTx) derived from scorpion Tityus serrulatus, it was possible to visualise KV1.2 subunits of the molecular layer and Purkinje cells of the rat cerebellum, including the pinceau regions of basket cells, confirming TsTx targeting the basket cell axon terminals and Purkinje cell dendrites [55]. Finally, the selectivity of scorpion Leiurus quinquestriatus hebraeus derived agitoxin-2 (AgTx-2) for KV1.3 subunit enabled visualisation of channels containing this protein in transfected HEK293 cells, using TAMRA labelling (AgTx-2-D19C-TAMRA). Through confocal imaging, the surface expression of KV1.3 in HEK293 cells was confirmed (https://www.alomone.com/p/agitoxin-2-cys-tamra/RTA-420-T). Another study used AgTx-2 labelled with FITC and Cy3 to elucidate the relationship of KV2.1 and KV10.2 with actin filaments, as well as the distribution and clustering of KV1.1, KV2.1 and KV10.2 in transfected green monkey kidney Vero cells [56]. Whilst there was no association of actin filaments stained with Alexa488-phalloidin with KV2.1 channels, a strong co-localization of actin was observed with KV10.2, supporting differential sorting and trafficking of these channels through interactions with actin filaments [56]. The utility of quantum dot (QD)–tagged toxins for imaging KV1.3 has been also shown using margatoxin (MgTx) obtained from the venom of the scorpion Centruroides margaritatus [57, 66]. MgTx linked via amino group-carboxylic crosslinker with QD CdSe-ZnS core-shell nanocrystals showed high selectivity to KV1.3 [57]. To demonstrate the specificity and high quantum efficacy of QDs-MgTx, it was applied to HEK293 cells transfected with GFP tagged KV1.3 construct, and showed a strong co-localisation of QDs with GFP, yielding a more stable and stronger QD signal [57]. A very recent study showed that conjugation of the scorpion Heterometrus spinifer-derived HsTX1 analogue HsTX1 [R14A] with Cy5 allowed the visualisation of GFP-KV1.3 channels in CHO cells [58]. Cy5-HsTX1 enabled also imaging of Kv1.3 in BV-2 microglia cells of C57BL/6 mice treated with lipopolysaccharides (LPS), known to upregulate KV1.3 subunit. The presence of fluorescence signals in the kidney, intestine and liver of C57BL/6 mice injected with Cy5-HsTX1 [R14A] implies its potential usefulness for biodistribution studies. Authors suggest that Cy5-HsTX1 [R14A] is a useful probe to determine the location and distribution of KV1.3 channels under physiological as well as autoimmune and neuroinflammatory conditions, associated with the upregulation of KV1.3 channels [58].
Table 1.
Optical probes targeting potassium channels
| Target, ion channel | Ligand (toxin) | Fluor-reporter | Model | Ref. |
|---|---|---|---|---|
| KV1.1, KV1.2 | HgTx1 | Cy3, Alexa488, 546 | Cerebellum, rabbit | [52] |
| KV1.3 | HgTx1 | Cy5 | Jurkat cell line | [53] |
| KV1.3 | HgTx1 | Cy5 | Jurkat cell line | [54] |
| KV1.2 | TsTx | ATTO594 | Cerebellum, rat | [55] |
| KV2.1, KV10.2 | AgTx-2 | FITC | Vero cells | [56] |
| KV1.1 | AgTx-2 | Cy3 | Vero cells | [56] |
| KV1.3 | MgTx | Quantum dots | HEK293 cells | [57] |
| Kv1.3 | HsTx1 | Cy5 | CHO and BV-2 cells, mouse | [58] |
| KV1.3 | AgTx-2 | TAMRA | HEK293 cells | AL |
| KV1.1, KcsA-KV1.1 | HgTx1 | ATTO488 | E. coli | [59] |
| KV1.3, KcsA- KV1.3 | AgTx-2 | TAMRA | E. coli | [60] |
| KcsA-KV1,x (x;1,3,6) | AgTx-2 | Tag-RFP | E. coli | [61] |
| KcsA-KV1.x (x;1,3,6) | OSK1 | eGFP | E. coli | [61] |
| KV1.3, KcsA- KV1.3 | AgTx-2 | GFP | E. coli | [62] |
| KV1.1, KcsA-KV1.1 | HgTx1 | Tag-RFP | E. coli | [63] |
| KV1.1, KcsA- KV1.1, KcsA- KV1.3 | HgTx1 | Tag-RFP | E. coli | [64] |
| KV1.3, KcsA- KV1.3 | MgTx | GFP | E. coli | [65] |
| KV2.1 | GxTx | Dye550 | CHO-K1 cells | [70] |
| KV2.1 | GxTx | TMR | Hippocampus, rat | [70] |
| KV2.1 | GxTx | Alexa594 | CHO cells, hippocampus, rat | [71] |
| BKCa | IbTx | Alexa488 | HEK293 cells | [72] |
| BKCa | IbTx | Alexa488 | Cochlear hair cells, mouse | [73] |
| BKCa | ChTx | Texas red | Neuromusc. junction, frog | [74] |
| SKCa | Apamin | Alexa488, 546 | Hippocampal, rat | [75] |
| SKCa | Apamin | Alexa488, 546 | Hippocampus, rat | [76] |
| Kir3.1, Kir3.4 | Tertiapin-Q | ATTO488 | HEK293 cells | [77] |
| Kir1.1 | Tertiapin-Q | FTIC-AuNP | PC12 cells | [78] |
Fig. 2.
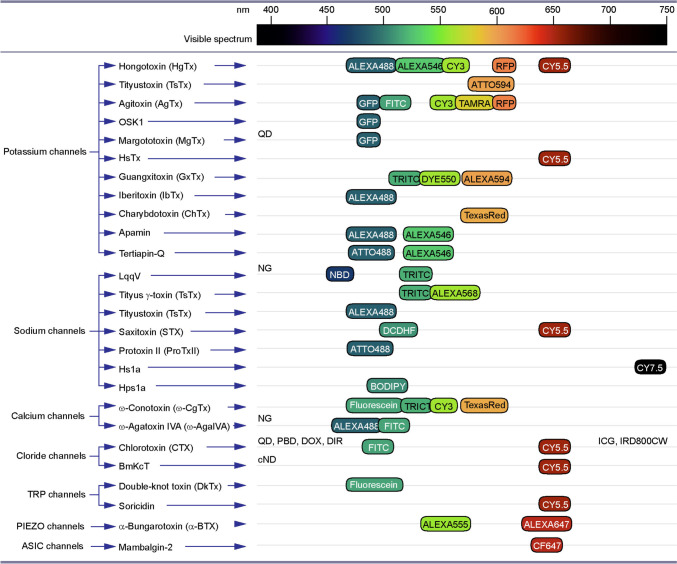
Animal toxin-derived optical probes targeting ion channels for biomedical research and clinical application. List of ion channel targets (left column) of animal toxins (middle column) used for research tagged with fluorophores, reporter proteins and nanomaterials (right column). Fluorophores and reporter proteins are aligned against the visible spectrum bar (top) to illustrate their peak absorbance. Fluorescence labels and reporters used for targeted toxins with a wide absorbance range or with a peak absorbance outside of the visible spectrum are listed on the left and right margins of the right column, respectively. QD, quantum dots; NG, nanogold; PBD, polymer-blend dots; DOX, doxorubicin; DIR, dye R; cFND, fluorescent nanodiamonds with carboxyl; ICG, indocyanine green; IRD800CW, infrared dye 800CW
Recombinant KV Channels with Shaker Channel Binding Domains
In addition to visualising native channels, venom toxins were used also for imaging chimeric K+ channel proteins (KcsA-KV1,x; x=1,3,6) made by transfer of the eukaryotic (KV) S5-S6 linker region within the pore domain in bacterial homologues (KcsA) [67]. The specific binding of ATTO488-labelled HgTx [59] and TAMRA-tagged AgTx-2 [60] with KcsA-KV1.1 and KcsA-KV1.3 chimaeras expressed in E. coli spheroplasts enabled their visualisation using confocal imaging. Likewise, in E. coli with and without recombinant KcsA-KV1.x (1,3,6), OSK1-eGFP (toxin derived from scorpion Orthochirus scrobiculosus) and AgTx-2-TagRFP were reported to be effective for selective labelling KV1 channels [61]. Notably, AgTx-2 with GFP at its N-terminus exhibits high specificity for chimeric KV1.3 channels over KV1.1 and KV1.6 in spheroplasts, and KcsA-KV1.3 transfected HEK293 cells [62]. Similar studies with the fusion of HgTx with Tag-RFP showed maintained specificity and utility for targeting KcsA-KV1.1 [63] as well as both KcsA-KV1.1 and KcsA-KV1.3 [64]. Finally, GFP-MgTx fusion protein revealed specific labelling of the KV1.3 subunits of the KcsA-KV1.3 hybrid channels expressed in E. coli spheroplasts, whilst control experiments with non-transfected material showed no fluorescence signal [65].
Shab K+ Channels
Two members of this family, KV2.1 and KV2.2 multimerize to form functional channels mediating delayed rectifier currents [68, 69]. Genetically modified (S13C) and fluor-labelled guangxitoxin (GxTX) variants from tarantula Plesiophrictus guangxiensis venom were used to study KV2.1 channels of transfected CHO cells and visualise endogenous KV2.1 channels of rat hippocampal pyramidal neurons [70, 71]. Tetramethylrhodamine (TMR, known also as TRITC) and Dye550-labelled GxTx enabled imaging of KV2.1 channel localization and expression, with binding activity depending on voltage changes in CHO-K1 cells [71], whilst Alexa Fluor 594-GxTx has been used to visualise the expression of KV2.1 in transfected CHO cells, demonstrating a correlation of fluorescence signal with membrane voltage. Also, Alexa594-GxTx enabled surface labelling of KV2.1-GFP transfected rat CA1 pyramidal neurons [70].
Ca2+-Activated K+ Channels
For mapping of big conductance calcium-activated potassium channels (BKCa) on the surface of living HEK293 cells, the venom of scorpion Mesobuthus tamulus iberiotoxin (IbTx) has been chemically modified and linked with Alexa488. Alexa488-IbTx showed a strong signal on the membrane surface of BKCa-expressing HEK293 cells, whereas no fluorescence was observed in non-transfected HEK293 cells [72]. Application of a recombinant iberiotoxin (IbTx-D19C) tagged with Alexa488 in mouse inner hair cells revealed the strong expression of BKCa [73]. Earlier work with the use of a similar approach showed the utility of the Streptavidin-Texas Red-labelled biotinylated-charybdotoxin (ChTx) derived from the venom of scorpion Leiurus quinquestriatus hebraeus as an imaging probe for BKCa channels, which were enriched in proximity to presynaptic Ca2+ channels at synaptic terminals of frog neuromuscular junctions (NMJ) [74]. Abiraman and co-workers used Alexa488- and Alexa546-streptavidin-tagged apamin isolated from the bee venom to map the distributions of Small Conductance KCa channels (SKCa+2) in somatodendritic compartments of hippocampal neurons in culture [75]. Imaging of channels with fluor-labelled biotinylated-apamin confirmed the hypothesis that SK channels of hippocampal neurons are enriched in the initial segment of axons [76].
Inward Rectifier K+ Channels
Isolated from honeybee venom, tertiapin-Q shows high selectivity for inward rectifier K+ (Kir) channels. Fluor-labelled tertiapin-Q-ATTO488 was used as a tool to visualise the localization and monitor the dynamics of Kir3.1 and Kir3.4 in HEK293 cells in a study of the effects of small molecule (drug chloroquine) blocker of Kir channels in atrial fibrillation [77]. Another example of the successful use of fluor-labelled tertiapin-Q is FITC fluorescein-labelled gold nanoparticle conjugated with tertiapin-Q (TPN-Q-AuNP/FITC), which helped to visualise the Kir1.1 channel upregulation in pheochromocytoma cell (PC12) by nerve growth factor (NGF) [78]. Results of imaging studies confirmed the specificity of TPN-Q-AuNP to various Kir channels in PC12 cells, with the potential utility for a wide range of research applications.
Optical Probes Targeting Sodium Channels
The sodium channel family is the second most diverse group of ion channels, playing a key role in membrane excitability, and the generation and propagation of action potentials in neurons, muscle cells and other excitable tissue [79, 80]. Because of the critical importance of Na+ channels in cellular activity, they have been a favourite target of many biological toxins [81, 82], which present significant interest as probes for selective labelling of various cell types and their functional processes, as well as for delivery of therapeutic candidates (Table 2, Fig. 2).
Table 2.
Optical probes targeting sodium channels
| Target, ion channel | Ligand (toxin) | Fluor-reporter | Model | Ref. |
|---|---|---|---|---|
| NaV | LqqV | DNB | Sciatic nerve, mouse | [87] |
| NaV | LqqV | TmRhd | Myotubes, chick | [88] |
| NaV | LqqV | TmRhd | Spinal cord, rat | [89] |
| NaV | TiTx- γ | TmRhd | Cortex, rat | [89] |
| NaV | TiTx- γ | TmRhd | Dorsal root ganglion, rat | [90] |
| NaV | TiTx- γ | Alexa568 | GH3 cells | [91] |
| NaV | TsTx | Alexa488 | GH3 cells | [91] |
| NaV | STX | Cy5 | PC12 cells | [92] |
| NaV | STX | DCDHF | PC12 cells | [92] |
| NaV1.7 | ProTx II | ATTO488 | CHO cells | [93] |
| NaV1.7 | Hsp1a | BODIPY | Sciatic nerve, mouse | [94] |
| NaV1.7 | Hs1a | Cy7.5 | HEK293 cells | [95] |
Pan-Na Channels
Animal toxins have been critical in the characterisation of the structure and function of Na+ channels [83, 84], with tetrodotoxin (TTX) and saxitoxin (STX) playing an important role in describing the channel properties, structure of the selective filter and toxin binding [85, 86]. A wide variety of animal toxins targeting Na+ channels have been described, with some successfully used for imaging. Angelides and Nutter were the first to use fluorescence tetrodotoxin of pufferfish Takifugu obscurus, and 2,6-dimethylnitrobenzene (DNB) conjugated LqqV toxin of scorpion Leiurus quinquestriatus venom to target Na+ channels, mapping their expression in nodal regions of myelinated fibres of mouse sciatic nerves, enhanced by immunohistochemical staining [87]. A follow-up report with tetramethylrhodamine dextran (TmRhd)-LqqV showed enrichment of Na+ channels at the NMJ of embryonic chick myotubes with co-cultured spinal cord neurons [88]. To identify regions where Na+ channels are densely expressed, neurons were treated with neurotoxin conjugates NBD-LqqV, TmRhd-LqqV, CPM-Css II, NBD-TTX and TmRhd-Tityus-γ [89]. It was found that in cortical neurons, Na+ channels are enriched in the neuronal soma and reach the highest density in the axon hillock, in agreement with the latter as the site of initiation of action potentials. Another study investigated the mobility of sodium channels during myelination, using TmRhd-Tityus-γ application to cultured dorsal root ganglion neurons in the presence and absence of Schwann cells (SCs), which change the distribution of Na+ channels in axons from diffuse to clustered in the nodal region [90]. Fluorescence photobleaching recovery measurements in this model showed that SCs did not affect sodium channel lateral mobility in the membrane.
In addition to labelling neurons, TsTx (α-type) and TiTX-γ (β-type) of scorpion Tityus serrulatus venom conjugated with Alexa488 and Alexa568 fluorophores, respectively, were used to target NaV channels in living GH3 cells, to visualise their distribution [91]. The specificity of labelling was proven by the preincubation of cells with naive toxins or treatment of non-transfected HEK293 cells with the labelled toxin. Like in Tityus serrulatus toxins studies, STX of paralytic shellfish poison labelled with N-hydroxysuccinimde (NHS) derivatives of Cy5 and DCDHF fluorophores enabled mapping of NaV distributions in NGF differentiated PC12 cells, with antibody labelling of Na+ channels verifying the staining specificity [92]. Finally, STX-Cy5 allowed the visualisation of channel distributions in living cells at the single molecule level, whilst the use of super-resolution methods made possible STX-Cy5 imaging of neuritic spines and filopodia in NGF-differentiated PC12 cells [92].
NaV1.7 Channels
To visualise surface expression and distribution of NaV1.7 channels in neurons, fluorescence labelling of Protoxin II (ProTx II) from the venom of tarantula spider Thrixopelma pruriens with selectivity to NaV1.7 was used [93]. ATTO488-tagged ProTxII was applied on CHO cells expressing human NaV1.7, on naïve CHO cells lacking NaV1.7 channels, and on dorsal root ganglion (DRG) neurons expressing endogenous NaV1.7. These experiments resulted in the labelling of NaV1.7 channel expressing CHO cells, as well as the proximal neurite parts and soma of DRG neurons [93]. The inability of ATTO488-ProTxII to bind labeled CHO cells pre-incubated with non-labelled ProTxII proved the toxin specificity for NaV1.7 channels. Another report used BODIPY-FL-NHS ester dye-conjugated Hsp1a peptide toxin from the venom of the tarantula Homoeomma spec, known for its selectivity for NaV1.7 channels [94]. Injection of this probe in mice resulted in high-intensity fluorescence signals of sciatic nerves tested in vivo and ex vivo [94]. Similarly, labelling a recombinant peptide analogue of Hs1a isolated from the venom of the Chinese bird spider Haplopelma schmidti with the Cy7.5 made visible NaV1.7 expressed in mouse sciatic nerves and enabled their near-infrared imaging in nodes of Ranvier [95].
Optical Probes Targeting Calcium Channels
Calcium channels are a family of proteins forming Ca2+ selective pores, which play a key role in a wide range of biochemical and electrophysiological processes in neurons and other cells, including control of molecular signalling, regulation of secretory processes, excitability and action potentials, gene regulation and others [96, 97]. Like other prevalent ion channels, Ca+2 channels have been a major target for a range of biological toxins [36, 98]. Due to the high affinity and selectivity of some of the toxins for Ca+2 channels, their labelling has been utilized for studies of channel distribution and function in situ and heterologous expression models (Table 3, Fig. 2).
Table 3.
Optical probes targeting calcium channels
| Target, ion channel | Ligand (toxin) | Fluor-reporter | Model | Ref. |
|---|---|---|---|---|
| P/Q-type | ω-Aga IVA | FTIC | Hippocampus and cerebellum, mouse | [102] |
| P/Q-type | ω-Aga IVA | Alexa488-nanogold | Brainstem, mouse | [103] |
| N-type | ω-CgTx | TmRdh | Hippocampus, rat | [104] |
| N-type | ω-CgTx | TexasRed | Neuromuscular junction, frog | [105] |
| N-type | ω-CgTx | TmRhd | Motor nerve, frog | [106] |
| N-type | ω-CgTx | TmRhd | Cerebellum, mouse | [107] |
| N-type | ω-CgTx | Fluorescein | Hippocampus, rat | [108] |
| N-type | ω-CgTx | Fluorescein | Hippocampus, rat | [109] |
| N-type | ω-CgTx | Cy3 | Hippocampus, rat | [110] |
P/Q Type Channels
Derived from the funnel-web spider Agelenopsis apera venom, ω-agatoxin IVA (ω-Aga IVA) has superb affinity and selectivity for P/Q type voltage-gated Ca2+ channels [99–101]. Using confocal microscopy of mouse brain tissue, biotinylated ω-Aga IVA labelled with FITC-avidin D enabled mapping of the distribution of P/Q Ca2+ channels in the mouse brain, particularly in the cerebellum and hippocampal region [102]. Based on the imaging results, it was concluded that P/Q Ca2+ channels are expressed in soma and dendrites of Purkinje cells, granule cells and interneurons in the cerebellum, as well as pyramidal cells of the CA1 and CA4 regions of the hippocampus. These results agree with electrophysiological data on P/Q-type Ca2+ channels in described neuron types and brain regions. Biotinylated ω-Aga IVA tagged with Alexa Fluor488-streptavidin nanogold (NG) was also applied to mouse auditory brainstem slices, demonstrating distinctive labelling of binding sites [103]. High-power microscopic analysis allowed visualisation of the distribution of P/Q channels, with no labelling observed in slices treated with Alexa Fluor488-streptavidin nanogold alone in P/Q channel knockout mice, confirming the selectivity of the probe [103].
N-Type Channels
Like ω-Aga IVA, labelled ω-conotoxin (ω-CgTx) of the venom of Pacific cone snail Conus geographus has been used for describing the distribution of N-type Ca2+ channels in cells. In hippocampal CA1 neurons of rats, the application of ω-CgTx labelled with a colloidal gold particle and TmRdh enabled visualisation of N-type Ca2+ channels of the soma and dendrites of neurons with clustering on synaptic terminals [104]. The feasibility of imaging of Ca2+ channels in nerve terminals of frog NMJ and their interactions with acetylcholine receptors was shown using ω-CgTx conjugated with Texas red [105]. It was found that Ca2+ channels at NMJ colocalize with acetylcholine receptors, verified also by α-bungarotoxin staining. Similarly, conjugation of ω-CgTx with the succinimidyl ester of TmRhD strongly labelled Ca2+ channels clustered at active zones of presynaptic terminals in frog motor nerves [106].
Microscopic analysis with TmRhd-ω-CgTx revealed the presence of N-type Ca2+ channels on the surface of postmitotic granule cells in developing mouse cerebellar slices, with their expression maintained in migrating and maturating neurons [107]. Studies of living rat hippocampal brain slices labelled with fluorescein-ω-CgTx conjugate allowed visualisation of N-type Ca2+ channels in all hippocampal regions, along with their distribution in various neuronal compartments and dendritic spines [108]. The distribution of N-type Ca2+ channels was also analysed during the development of rat brains [109]. Finally, the same approach using ω-CgTx-Cy3 allowed the detection of the changes in the expression of N-type Ca2+ channels in hippocampal slices of the kindling model of rat epilepsy [110]. Analysis of the labelling of dendrites of CA1 and CA3 neurons in epilepsy rat models with CgTx-Cy3 showed alterations in signal intensity compared to controls, suggesting changes in the prevalence of N-type Ca2+ channel in epilepsy disorders.
Optical Probes Targeting Chloride Channels
Chloride channels are a functionally and structurally diverse group of anion-selective proteins involved in the regulation of a range of functions in cells, including membrane potential, cell volume and cycle and apoptosis [111, 112]. Cl- channels are classified into several subfamilies [113], which are differentially expressed in various cell types under physiological and disease conditions [114, 115]. Like K+ and other cation channels, specific enrichment of some Cl- channels has been observed in neoplastic tissue, which renders them useful for molecular imaging and targeting therapies.
Small Conductance Chloride Ion Channels
Chlorotoxin (CTX) of the deathstalker scorpion Leiurus quinquestriatus [116, 117] is a selective ligand for Cl- channels, also known to bind matrix metalloproteinase-2 (MMP-2) and annexin-2 on the cell membrane [118–120] (Table 4, Fig. 2). Cy5.5 conjugation with NHS ester to CTX allowed imaging of the 9L rat glioma cells in vitro and glioma xenograft mice, as well as in brain slices of ND2:SmoA1 medulloblastoma model [121]. The specificity of labelling was verified using a competitive assay with nonlabelled CTX. CTX-Cy5.5 also enabled imaging of prostate cancer in epithelium and lymph nodes, lung metastases and sarcoma in mouse cancer models. Substitution of lysine 15 and lysine 23 residues of CTX (K15A K23ACTX:Cy5.5 or K15R K23RCTX:Cy5.5) allowed prevention of mono-, di-, tri-labelling, which causes complications with quantitative imaging, and enables mono- lysine 27 labelling [122]. In the same report, cyclized CTX has been developed and used to optimise the serum stability of CTX bioconjugates [122]. It has been found that substituted and cyclized Cy5.5 labelled CTX retained the specificity and displayed the ability to selectively label tumour regions in ND2:SmoA1 medulloblastoma models [122].
Table 4.
Optical probes targeting chloride channels
| Target, ion channel | Ligand (toxin) | Fluor-reporter | Model | Ref. |
|---|---|---|---|---|
| MMP-2 (Cl-) | CTX | Cy5.5 | 9L cells (in vitro); glioma, prostate, lung metastases xenograft mice, ND2SmoA1 medulloblastoma mice | [121] |
| MMP-2 (Cl-) | CTX | Cy5.5 | ND2:SmoA1 medulloblastoma mice | [122] |
| MMP-2 (Cl-) | CTX | ICG | LN229 xenograft mice | [123] |
| MMP-2 (Cl-) | CTX | ICG | Dog models with various solid tumours | [125] |
| MMP-2 (Cl-) | CTX | ICG | Head and neck squamous carcinoma | [126] |
| MMP-2 (Cl-) | CTX | ICG | Human skin cancer lesions | [127] |
| MMP-2 (Cl-) | CTX | IRD-800CW | U87MG xenograft mice, NS2:SmoA1 mice | [128] |
| MMP-2 (Cl-) | CTX | PEG-iron oxide-Cy5.5 | ND2:SmoA1 medulloblastoma mice | [129] |
| MMP-2 (Cl-) | CTX | Magnetic iron oxide-FTIC | U251MG, C6 cells | [130] |
| MMP-2 (Cl-) | CTX | NaYF(4):Yb,Er/Ce | C6 cells, C6 xenograft mice | [131] |
| MMP-2 (Cl-) | CTX | DOX-liposomes | U251MG, U87 and C6 cells | [132] |
| MMP-2 (Cl-) | CTX | Dir-liposomes | U87, xenograft mouse | [132] |
| MMP-2 (Cl-) | CTX | DOX-liposomes | 4T1 cells | [133] |
| MMP-2 (Cl-) | CTX | Dir-liposomes | 4T1, xenograft mice | [133] |
| MMP-2 (Cl-) | CTX | AIS/Zns QD/PLGA-PEG MI | U87 cells | [134] |
| MMP-2 (Cl-) | CTX | C-6 loaded PLGA, nano | GI-1, U87 cells | [135] |
| MMP-2 (Cl-) | CTX | Nano-agent, ICG | U87MG, xenograft mouse | [136] |
| MMP-2 (Cl-) | CTX | Polymer-blend dot | ND2:SmoA1 medulloblastoma mice | [137] |
| MMP-2 GCC | BmKcT | Cy5.5 | C6 cells | [142] |
| MMP-2 GCC | BmKcT | Carboxylated-ND | C6 cells | [143] |
The utility of peptide fluorophore CTX-indocyanine green (ICG), known also as BLZ-100, for surgical guidance and resection of malignant tissue was reported by numerous studies [123, 124]. BLZ-100 accumulated in the neoplastic area of LN229 human glioblastoma grafted mice, allowing NIR imaging, whereas ICG alone did not produce specific labelling of the pathological tissue [123]. The ability of BLZ-100 to differentiate healthy and malignant tissue was tested also in dog models of tumours [125]. Baik and co-workers used BLZ-100 for imaging mouse xenografts of head and neck squamous cell carcinoma (HCSCC) and hamster xenografts of high- and low-risk oral dysplasia [126]. In PCI-15B xenografts expressing GFP, uptake of free ICG alone did not produce fluorescent signals, confirming the tumour-specificity of BLZ-100. Recently, human clinical trials of BLZ-100 were performed in patients with skin cancer, demonstrating specific and concentration-dependent labelling of neoplastic tissue [127]. In neuroimaging applications, CTX conjugated with infrared dye 800 CW (IRD-800CW) labelled the U87MG glioblastoma xenografts in mice, strongly colocalizing with MMP-2 expression [128]. In the same study, brain slices of ND2:SmoA1 genetically modified medulloblastoma mice treated with IRD-800CW-CTX exhibited a higher fluorescence than in tumour-free mice [128]. Using PEG-iron oxide-CTX-Cy5.5 nanoprobes as NIR optical imaging tool, Veiseh and co-workers visualised medulloblastoma in ND2:SmoA1 model mice, with the utility for intraoperative tumour resection verified through histological analysis [129].
Meng and co-workers showed that CTX functionalized iron oxide nanoparticles linked with FITC allow the labelling of U251MG and C6 rat glioma cells [130]. Another example of functionalizing nanoparticles with CTX for imaging was the use of hexagonal-phase NaYF(4):Yb,Er/Ce (up-conversion, UCNP) labelling of root C6 rat glioma cells, which showed that in addition to enhanced accumulation of CTX-UCNP nanoprobe, the signal was specifically localised to the tumour region of C6 glioma-bearing xenograft mice [131]. Successful use of CTX-targeted liposomes (LP) has been also shown for the delivery of fluorescence load to glioma cells, allowing their visualisation with optical imaging [132]. In U251MG, U87 human and C6 rat glioma cell lines, doxorubicin (DOX)-loaded CTX-LP emitted a stronger fluorescence signal compared to non-targeted DOX-loaded LP. Unlike free DIR and blank LP, targeted DIR-loaded LP showed enhanced accumulation in U87 cell-bearing armpit and orthopaedic mouse tumour models, including in brains [132]. DOX-LP functionalised with CTX (DOX-SSL-CTX) was also utilized as an imaging probe in 4T1 murine breast cancer cells, to evaluate the uptake of liposomes [133]. As a result, higher fluorescence intensity was observed with DOX-SSL-CTX compared with CTX-free DOX-SSL. Finally, modification of Dir-loaded liposomes with CTX (Dir-SSL-CTX) was used to investigate the targeting efficiency of liposomes in 4T1 cells bearing BALB/c mice in vitro. Authors conclude that CTX enhances the LP targeting and cargo internalisation in metastatic breast tumours [133].
CTX has been also successfully utilised for functionalizing AIS/ZnS quantum dots (QD) with Polylactide-co-glycolide (PLGA)-polyethylene glycol (PEG) micelles to U87 brain tumour cells [134]. Pre-treatment of the U87 cells with MMP-2 inhibitor before AIS/ZnS QDs treatment resulted in a decrease in fluorescence signal. These results demonstrated the effectiveness of CTX as a tool for targeted imaging of biological specimens using AIS/ZnS QDs. CTX has also been shown to enhance the targeting of PLGA nanoparticles loaded with anti-cancer mourisin (MOR) to glioma cells [135]. Nanoparticles loaded with coumarin-6 (C-6) conjugated to CTX (instead of MOR) and free nanoparticles were applied in HCN-1A, GI-1 and U87 cells in culture. Results of these studies show that whilst human neuronal cell line HCN-1A displayed a weak fluorescence signal due to lack of MMP-2 receptor, the fluorescence signal was stronger in glioma GI-1 and U87 cell lines. Patil and co-workers developed polymalic acid (PLMA) nanoparticle platform with tri-leucine peptide (LLL) and ICG, with CTX used for their targeting [136]. Selective labelling of U87MG glioma-bearing xenograft by PLMA-LLL-ICG-CTX was observed in a mouse brain, but not in tumour-free tissue. CTX and tri-leucine peptide-free PLGA-ICG showed little or no signal, whilst tri-leucine peptide-free agents showed a lower signal than PLMA-LLL-ICG-CTX [136]. Finally, Wu and co-workers applied CTX targeting to polymer-blend dots (Pbdot, PBD), which are ~15 times brighter than QDs at 655nm and show significantly higher stability in serum, with no toxicity. Authors report strong and specific Pdot-CTX ND2:SmoA1 labelling of medulloblastoma of mouse brain ex vivo. Contrary to this, there was no signal in ND2:SmoA1 with control Pbdot-PEG and in C57BL/6 wild-type mice lacking brain tumours [137].
Glia-Specific Cl- Channel
There have been reports of the expression of a specific Cl- channel (GCC) in human astrocytoma cells, which could be blocked by CTX [138, 139]. Some studies have shown that chlorotoxin-like toxin BmKcT derived from the cDNA of the Chinese scorpion Mesobuthus martensii Karsch's salivary glands bind specifically to this channel [140, 141]. Like CTX, Cy5.5 conjugated BmKcT was tested in a glioma rat model produced by grafting C6 cells [142] (Fig. 2). BmKcT targeting caused a much stronger labelling of tumour tissue compared to non-targeted Cy5.5 [142]. Another report tested BmKCT functionalized fluorescent nanodiamonds with carboxyl (cFND) in C6 cells, and showed stronger labelling of the malignant tissue, compared to non-targeted cFND [143].
Optical Probes Targeting TRP Channels
Over 30 transient receptor potential (TRP) channel homologues have been identified in mammals, which are divided into six subfamilies [144]. One of these, TRPV consists of six members: TRPV1-TRPV6. TRPV1-TRPV4 are enriched in the sensory system, playing a key role in detecting various stimuli such as heat, stretch, acidity and pain, and have low selectivity for Ca2+, whilst TRPV5-6 are highly selective for Ca2+ and regulate molecular signalling and housekeeping functions in a wide variety of cells [145–150].
TRPV1 Channels
To visualise the expression and distribution of TRPV1 channels, double-knot-toxin (DkTx) from tarantula Ornithoctonus huwena venom [151] conjugated with fluorescein via sortase at the C-terminus was applied to Xenopus leaves oocytes transfected with rat TRPV1 channels [152] (Table 5; Fig. 2). Because DkTx contains a large number of cysteine residues, cysteine-mediated bioconjugation caused a high level of misfolding of the toxin. Nevertheless, the authors report that their approach enabled successful and selective labelling of rat TRPV1 in the oocyte model [152].
Table 5.
Optical probes targeting TRPV1, PIEZO1 and ASIC1a channels
TRPV6 Channels
TRPV6 has been implicated in neoplasia, with its level enhanced in the ovary, breast, colon, prostate and thyroid cancers [153, 154]. In prostate cancer, TRPV6 mRNA levels are positively correlated to tumour progression and aggressiveness, pathological stages and extra-prostatic metastases, with poor prognosis [155, 156]. Bowen and co-workers used soricidin (accession number P0C2P6)—a paralytic peptide isolated from the submaxillary saliva glands of the northern short-tailed shrew Blarina brevicauda, known to inhibit Ca2+ influx via TRPV6 channels [157]. Two C-terminus peptide sequences of soricidin (SOR-C13 and SOR-C27) were shown to bind specifically TRPV6 in ovarian cancer cells [157] as well as ovarian cancer xenografts in mouse models. Conjugation of C-terminus SOR-27 peptide sequence of soricidin with Cy5.5 and superparamagnetic iron oxide, when applied in vivo, accumulated in the ovarian tumour regions of xenograft mice and could be visualised with fluorescence imaging [157]. Authors conclude that SOR peptides may be useful for detecting tumours and delivering diagnostic or therapeutic payloads, via targeting TRPV6 channels.
Optical Probes Targeting Other Ion Channels
In addition to demonstrating the successful use of labelled toxins derived from animal venom for visualisation of main cation and anion-selective channels, some of the toxins were recently also successfully utilised as probes for imaging acid-sensing ion channels (ASIC) and Piezo receptor channels [158–160] (Table 5, Fig. 2).
Piezo1 Channel
To visualise the distribution and activity of mechanoreceptive channels, Lee and co-workers tailored recombinant Piezo1 protein containing α-bungarotoxin (α-BTX) binding site, which was visualised by α-BTX conjugated with Alexa555 (BTX-Alexa555) [159]. Exposure of N2A cells transfected with Piezo1-13 residue BTX binding site to Alexa555-BTX revealed strong and specific labelling. The construct has been used for addressing functional questions related to trafficking and regulation of Piezo1/2 channels [159]. Another study used α-BTX-Alexa647 for visualising HEK293 cells transfected with Piezo1 channels containing α-BTX binding sites, to determine the mechanically sensitive domains of Piezo1 channels and their response to magnetic nanoparticles [160].
ASIC1a Channel
A recent report by Bychkov and co-workers used modified mambalgin-2 for imaging the expression and distribution of ASIC1a channels [158]. Derived from black mamba Dendroaspis polylepis venom, mambalgin-2 specifically and reversibly blocks the pathological upregulation of ASIC1a in melanoma cancer associated with acidification of tumour tissue [161]. When applied on metastatic skin melanoma mel P cells, mambalgin-2 with Leu32Ala mutation labelled with the CF647 dye revealed a strong presence of ASIC1a subunit as well as colocalization with epithelial Na+ channel family members (α-ENaC and γ-ENaC), an observation verified with immunofluorescence microscopy [162]. Using a mutant variant of mambalgin-2 with reduced binding activity for ASIC1a, it was confirmed that the principal molecular target of mambalgin-2 in melanoma cells is the ASIC1a subunit [158].
Summary and Future Directions
Over millennia, severe pain, intoxication and death were what drew our respect and curiosity to venomous animals. The lethal power of animal toxins demanded not only reverence, but more recently, also systematic research and understanding, which with scientific progress and technological advances facilitated the harnessing of their ability to treat an expanding range of conditions and diseases. The plethora of biologically active compounds produced by venomous animals capable of targeting receptors and ion channels, as shown here, are not only relevant to pharmacology and toxicology but also molecular imaging, offering means for tackling major research and translational questions.
Since the first demonstration of the block of action potentials by TTX and inhibition of NaV currents in nerves and muscles [163, 164], biological toxins have played an increasing role in elucidating important facets of the molecular biology and functions of ion channels and receptors, aiding their isolation, cloning and pharmacological characterisation [86, 165–167]. Combined with revolutionary advances in fluorescence probes and nanomaterials and imaging technologies, the expanding portfolio of neurotoxins and their detoxified forms have been recently applied for a wide range of applications for preclinical and clinical imaging (Fig. 3). As reviewed herein, advances in harnessing the potential of neurotoxins for biological imaging have also revealed considerable limitations. These are largely attributed to remarkable potency and harmful effects of toxins, owing to their specific interference with important biological functions of their targets, as well as challenges related to immunological incompatibility and bioavailability. Non-specific interactions with a wide range of other functional proteins, and potential off-site effects, are also of major concern, along with the release of harmful products of fluorophore degradation with phototoxicity. In addition to the limitations associated with biological factors, the widespread use of toxin-based optical probes is also restrained by the physical properties of light and its interactions with the specimen, resulting in attenuation of fluorescence signals, confining high-resolution optical interrogation to the surface of the biological specimen.
Fig. 3.
Representation of basic and translational research areas of the use of animal toxin-derived optical probes targeting ion channels. List of ion channels (left column) targeted by labelled animal toxins (middle column) utilised for various research and translational applications (red circle). The details of the applications of each optical probe are described in corresponding sections of the review
In summary, along with well-recognised ecological, evolutionary and therapeutic dimensions, the ultimate weapons of nature, animal venoms, are becoming of increasing research and diagnostic relevance. Harnessing the full potential of animal toxins and their detoxified forms for biomedical imaging, through systematic research and optimization, is anticipated to improve the efficacy and specificity of toxin-based optical probes utilized in future research.
Acknowledgements
The authors thank the (1) Innovation Fund Award 2022 from the University of Greenwich and the (2) Research Excellent Framework Program.
Author Contribution
Conception and design: PHE, SS, VN and SVO; drafting, revising and critical comments: PHE, SS, VN and SVO; illustrations: PHE, SS, VN and SVO; review and approval of the final version: PHE, SS, VN and SVO.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010;7:603–614. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 2.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 3.Hillman EM (2007) Optical brain imaging in vivo: techniques and applications from animal to man. J Biomed Opt 12(5):051402. 10.1117/1.2789693 [DOI] [PMC free article] [PubMed]
- 4.Yoon S, Cheon SY, Park S et al (2022) Recent advances in optical imaging through deep tissue: imaging probes and techniques. Biomater Res 26 [DOI] [PMC free article] [PubMed]
- 5.Hellebust A, Richards-Kortum R. Advances in molecular imaging: targeted optical contrast agents for cancer diagnostics. Nanomedicine (London) 2012;7:429–445. doi: 10.2217/nnm.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Y, Annala AJ, Barrio JR, et al. Quantification of target gene expression by imaging reporter gene expression in living animals. Nat Med. 2000;6:933–937. doi: 10.1038/78704. [DOI] [PubMed] [Google Scholar]
- 7.Shemesh OA, Linghu C, Piatkevich KD, et al. Precision calcium imaging of dense neural populations via a cell-body-targeted calcium indicator. Neuron. 2020;107(470-486):e411. doi: 10.1016/j.neuron.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linghu C, Johnson SL, Valdes PA, et al. Spatial multiplexing of fluorescent reporters for imaging signaling network dynamics. Cell. 2020;183(1682-1698):e1624. doi: 10.1016/j.cell.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westphal V, Rizzoli SO, Lauterbach MA, Kamin D, Jahn R, Hell SW. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science. 2008;320:246–249. doi: 10.1126/science.1154228. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Suarez M, Ting AY. Fluorescent probes for super-resolution imaging in living cells. Nat Rev Mol Cell Biol. 2008;9:929–943. doi: 10.1038/nrm2531. [DOI] [PubMed] [Google Scholar]
- 11.Ntziachristos V, Razansky D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT) Chem Rev. 2010;110:2783–2794. doi: 10.1021/cr9002566. [DOI] [PubMed] [Google Scholar]
- 12.Lewis SM, Asselin-Labat ML, Nguyen Q, et al. Spatial omics and multiplexed imaging to explore cancer biology. Nat Methods. 2021;18:997–1012. doi: 10.1038/s41592-021-01203-6. [DOI] [PubMed] [Google Scholar]
- 13.Olefir I, Ghazaryan A, Yang H, et al. Spatial and spectral mapping and decomposition of neural dynamics and organization of the mouse brain with multispectral optoacoustic tomography. Cell Rep. 2019;26(2833-2846):e2833. doi: 10.1016/j.celrep.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ovsepian SV, Olefir I, Westmeyer G, Razansky D, Ntziachristos V. Pushing the boundaries of neuroimaging with optoacoustics. Neuron. 2017;96:966–988. doi: 10.1016/j.neuron.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Culver J, Akers W, Achilefu S. Multimodality molecular imaging with combined optical and SPECT/PET modalities. J Nucl Med. 2008;49:169–172. doi: 10.2967/jnumed.107.043331. [DOI] [PubMed] [Google Scholar]
- 16.Rivnay J, Wang H, Fenno L, Deisseroth K, Malliaras GG. Next-generation probes, particles, and proteins for neural interfacing. Sci Adv. 2017;3:e1601649. doi: 10.1126/sciadv.1601649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajasethupathy P, Ferenczi E, Deisseroth K. Targeting neural circuits. Cell. 2016;165:524–534. doi: 10.1016/j.cell.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gramlich MW, Klyachko VA. Nanoscale organization of vesicle release at central synapses. Trends Neurosci. 2019;42:425–437. doi: 10.1016/j.tins.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y, Li D, Zhang S, et al. Visualizing intracellular organelle and cytoskeletal interactions at nanoscale resolution on millisecond timescales. Cell. 2018;175(1430-1442):e1417. doi: 10.1016/j.cell.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 20.Skopintsev P, Ehrenberg D, Weinert T, et al. Femtosecond-to-millisecond structural changes in a light-driven sodium pump. Nature. 2020;583:314–318. doi: 10.1038/s41586-020-2307-8. [DOI] [PubMed] [Google Scholar]
- 21.Ntziachristos V. Fluorescence molecular imaging. Annu Rev Biomed Eng. 2006;8:1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- 22.Thiruppathi R, Mishra S, Ganapathy M, Padmanabhan P, Gulyas B. Nanoparticle functionalization and its potentials for molecular imaging. Adv Sci (Weinh) 2017;4:1600279. doi: 10.1002/advs.201600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thangam R, Paulmurugan R, Kang H (2022) Functionalized nanomaterials as tailored theranostic agents in brain imaging. Nanomaterials (Basel) 12(1):18. 10.3390/nano12010018 [DOI] [PMC free article] [PubMed]
- 24.Baetke SC, Lammers T, Kiessling F (2015) Applications of nanoparticles for diagnosis and therapy of cancer. Br J Radiol 88(1054):20150207. 10.1259/bjr.20150207 [DOI] [PMC free article] [PubMed]
- 25.Liu N, Mishra K, Stiel AC, Gujrati V, Ntziachristos V. The sound of drug delivery: optoacoustic imaging in pharmacology. Adv Drug Deliv Rev. 2022;189:114506. doi: 10.1016/j.addr.2022.114506. [DOI] [PubMed] [Google Scholar]
- 26.Mishra K, Fuenzalida-Werner JP, Pennacchietti F, et al. Genetically encoded photo-switchable molecular sensors for optoacoustic and super-resolution imaging. Nat Biotechnol. 2022;40:598–605. doi: 10.1038/s41587-021-01100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayvazyan NM, O'Leary VB, Dolly JO, Ovsepian SV. Neurobiology and therapeutic utility of neurotoxins targeting postsynaptic mechanisms of neuromuscular transmission. Drug Discov Today. 2019;24:1968–1984. doi: 10.1016/j.drudis.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Biswas A, Gomes A, Sengupta J, et al. Nanoparticle-conjugated animal venom-toxins and their possible therapeutic potential. J Venom Res. 2012;3:15–21. [PMC free article] [PubMed] [Google Scholar]
- 29.Edupuganti OP, Ovsepian SV, Wang J, et al. Targeted delivery into motor nerve terminals of inhibitors for SNARE-cleaving proteases via liposomes coupled to an atoxic botulinum neurotoxin. FEBS J. 2012;279:2555–2567. doi: 10.1111/j.1742-4658.2012.08638.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary VB, Ovsepian SV, Bodeker M, Dolly JO. Improved lentiviral transduction of ALS motoneurons in vivo via dual targeting. Mol Pharm. 2013;10:4195–4206. doi: 10.1021/mp400247t. [DOI] [PubMed] [Google Scholar]
- 31.Ovsepian SV, O'Leary VB, Ntziachristos V, Dolly JO. Circumventing brain barriers: nanovehicles for retroaxonal therapeutic delivery. Trends Mol Med. 2016;22:983–993. doi: 10.1016/j.molmed.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Jenner R, Undheim E. Venom: the secrets of nature’s deadliest weapon. CSIRO Publishing; 2017. [Google Scholar]
- 33.Wilcox C. Venomous: how Earth’s deadliest creatures mastered biochemistry. Farrar Straus and Giroux; 2017. [Google Scholar]
- 34.van Thiel J, Khan MA, Wouters RM, et al. Convergent evolution of toxin resistance in animals. Biol Rev Camb Philos Soc. 2022;97:1823–1843. doi: 10.1111/brv.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurenas D, Fraikin N, Goormaghtigh F, Van Melderen L. Biology and evolution of bacterial toxin-antitoxin systems. Nat Rev Microbiol. 2022;20:335–350. doi: 10.1038/s41579-021-00661-1. [DOI] [PubMed] [Google Scholar]
- 36.Ovsepian SV, O'Leary VB, Ayvazyan NM, Al-Sabi A, Ntziachristos V, Dolly JO. Neurobiology and therapeutic applications of neurotoxins targeting transmitter release. Pharmacol Ther. 2019;193:135–155. doi: 10.1016/j.pharmthera.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Popoff MR, Poulain B. Bacterial toxins and the nervous system: neurotoxins and multipotential toxins interacting with neuronal cells. Toxins (Basel) 2010;2:683–737. doi: 10.3390/toxins2040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivera-de-Torre E, Rimbault C, Jenkins TP, et al. Strategies for heterologous expression, synthesis, and purification of animal venom toxins. Front Bioeng Biotechnol. 2021;9:811905. doi: 10.3389/fbioe.2021.811905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Leary VB, Ovsepian SV, Raghunath A, et al. Innocuous full-length botulinum neurotoxin targets and promotes the expression of lentiviral vectors in central and autonomic neurons. Gene Ther. 2011;18:656–665. doi: 10.1038/gt.2011.8. [DOI] [PubMed] [Google Scholar]
- 40.Ovsepian SV, Bodeker M, O'Leary VB, Lawrence GW, Oliver Dolly J. Internalization and retrograde axonal trafficking of tetanus toxin in motor neurons and trans-synaptic propagation at central synapses exceed those of its C-terminal-binding fragments. Brain Struct Funct. 2015;220:1825–1838. doi: 10.1007/s00429-015-1004-0. [DOI] [PubMed] [Google Scholar]
- 41.Kim DM, Nimigean CM (2016) Voltage-gated potassium channels: a structural examination of selectivity and gating. Cold Spring Harb Perspect Biol 8(5):a029231. 10.1101/cshperspect.a029231 [DOI] [PMC free article] [PubMed]
- 42.Gutman GA, Chandy KG, Grissmer S, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 43.Huang X, Jan LY. Targeting potassium channels in cancer. J Cell Biol. 2014;206:151–162. doi: 10.1083/jcb.201404136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang F, Stournaras C. Ion channels in cancer: future perspectives and clinical potential. Philos Trans R Soc Lond Ser B Biol Sci. 2014;369:20130108. doi: 10.1098/rstb.2013.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ovsepian SV, LeBerre M, Steuber V, O'Leary VB, Leibold C, Oliver Dolly J. Distinctive role of KV1.1 subunit in the biology and functions of low threshold K(+) channels with implications for neurological disease. Pharmacol Ther. 2016;159:93–101. doi: 10.1016/j.pharmthera.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Laumonnier F, Roger S, Guerin P, et al. Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am J Psychiatry. 2006;163:1622–1629. doi: 10.1176/ajp.2006.163.9.1622. [DOI] [PubMed] [Google Scholar]
- 47.Wulff H, Zhorov BS. K+ channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem Rev. 2008;108:1744–1773. doi: 10.1021/cr078234p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ovsepian SV, Waxman SG. Gene therapy for chronic pain: emerging opportunities in target-rich peripheral nociceptors. Nat Rev Neurosci. 2023;24(4):252–265. doi: 10.1038/s41583-022-00673-7. [DOI] [PubMed] [Google Scholar]
- 49.Angulo E, Noe V, Casado V, et al. Up-regulation of the Kv3.4 potassium channel subunit in early stages of Alzheimer’s disease. J Neurochem. 2004;91:547–557. doi: 10.1111/j.1471-4159.2004.02771.x. [DOI] [PubMed] [Google Scholar]
- 50.Villa C, Suphesiz H, Combi R, Akyuz E. Potassium channels in the neuronal homeostasis and neurodegenerative pathways underlying Alzheimer’s disease: an update. Mech Ageing Dev. 2020;185:111197. doi: 10.1016/j.mad.2019.111197. [DOI] [PubMed] [Google Scholar]
- 51.Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992;72:S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- 52.Pragl B, Koschak A, Trieb M, et al. Synthesis, characterization, and application of cy-dye- and alexa-dye-labeled hongotoxin(1) analogues. The first high affinity fluorescence probes for voltage-gated K+ channels. Bioconjug Chem. 2002;13:416–425. doi: 10.1021/bc015543s. [DOI] [PubMed] [Google Scholar]
- 53.Schutz GJ, Pastushenko VP, Gruber HJ, Knaus HG, Pragl B, Schindler H. 3D imaging of individual ion channels in live cells at 40 nm resolution. Single Mol. 2000;1(1):25–31. doi: 10.1002/(SICI)1438-5171(200004)1:1<25::AID-SIMO25>3.0.CO;2-O. [DOI] [Google Scholar]
- 54.Freudenthaler G, Axmann M, Schindler H, Pragl B, Knaus HG, Schutz GJ. Ultrasensitive pharmacological characterisation of the voltage-gated potassium channel K(V)1.3 studied by single-molecule fluorescence microscopy. Histochem Cell Biol. 2002;117:197–202. doi: 10.1007/s00418-001-0374-y. [DOI] [PubMed] [Google Scholar]
- 55.Williams MR, Fuchs JR, Green JT, Morielli AD. Cellular mechanisms and behavioral consequences of Kv1.2 regulation in the rat cerebellum. J Neurosci. 2012;32:9228–9237. doi: 10.1523/JNEUROSCI.6504-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karlova MG, Pishchal'nikova AV, Ramonova AA, Moĭsenovich MM, Sokolova OS, Shaĭtan KV. In vitro fluorescence assay to study the folding of Kv ion channels. Biofizika. 2011;56(2):272–279. [PubMed] [Google Scholar]
- 57.Schwartz AB, Kapur A, Wang W, et al. Margatoxin-bound quantum dots as a novel inhibitor of the voltage-gated ion channel Kv1.3. J Neurochem. 2017;140:404–420. doi: 10.1111/jnc.13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wai DCC, Naseem MU, Mocsar G, et al. A fluorescent peptide toxin for selective visualization of the voltage-gated potassium channel K(V)1.3. Bioconjug Chem. 2022;33:2197–2212. doi: 10.1021/acs.bioconjchem.2c00436. [DOI] [PubMed] [Google Scholar]
- 59.Orlov N, Nekrasova O, Feofanov AV. Fluorescent ligands of Kv1 channels on the basis of hongotoxin: Atto488-hongotoxin. Microsc Microanal. 2019;25(S2):1278–1279. doi: 10.1017/S1431927619007128. [DOI] [Google Scholar]
- 60.Kudryashova KS, Nekrasova OV, Kuzmenkov AI, et al. Fluorescent system based on bacterial expression of hybrid KcsA channels designed for Kv1.3 ligand screening and study. Anal Bioanal Chem. 2013;405:2379–2389. doi: 10.1007/s00216-012-6655-6. [DOI] [PubMed] [Google Scholar]
- 61.Kuzmenkov AI, Nekrasova OV, Kudryashova KS, et al. Fluorescent protein-scorpion toxin chimera is a convenient molecular tool for studies of potassium channels. Sci Rep. 2016;6:33314. doi: 10.1038/srep33314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nekrasova OV, Primak AL, Ignatova AA, et al. N-terminal tagging with GFP enhances selectivity of agitoxin 2 to Kv1.3-channel binding site. Toxins (Basel) 12. 2020;12(12):802. doi: 10.3390/toxins12120802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skutel M, Primak A, Kirpichnikov M, Arseniev A, Feofanov A, Nekrasova O. RFP-tagged hongotoxin 1 and its interactions with KscA-Kv1.1 hybrid channels. Microsc Microanal. 2020;26(S2):1378–1380. doi: 10.1017/S1431927620017900. [DOI] [Google Scholar]
- 64.Primak AL, Skutel MA, Nekrasova OV. Kv1 potassium channel ligands based on hongotoxin 1 and red fluorescent protein. Russ J Bioorg Chem. 2020;46:1011–1017. doi: 10.1134/S1068162020060266. [DOI] [Google Scholar]
- 65.Denisova KR, Orlov NA, Yakimov SA, et al. GFP-margatoxin, a genetically encoded fluorescent ligand to probe affinity of Kv1.3 channel blockers. Int J Mol Sci. 2022;23:1724. doi: 10.3390/ijms23031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Calvo M, Leonard RJ, Novick J, et al. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem. 1993;268:18866–18874. doi: 10.1016/S0021-9258(17)46707-X. [DOI] [PubMed] [Google Scholar]
- 67.Kudryashova KS, Nekrasova OV, Kirpichnikov MP, Feofanov AV. Chimeras of KcsA and Kv1 as a bioengineering tool to study voltage-gated potassium channels and their ligands. Biochem Pharmacol. 2021;190:114646. doi: 10.1016/j.bcp.2021.114646. [DOI] [PubMed] [Google Scholar]
- 68.Islas LD, Sigworth FJ. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J Gen Physiol. 1999;114:723–742. doi: 10.1085/jgp.114.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005;26:743–752. doi: 10.1016/j.neuro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Thapa P, Stewart R, Sepela RJ, et al. EVAP: A two-photon imaging tool to study conformational changes in endogenous Kv2 channels in live tissues. J Gen Physiol. 2021;153:e202012858. doi: 10.1085/jgp.202012858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tilley DC, Eum KS, Fletcher-Taylor S, et al. Chemoselective tarantula toxins report voltage activation of wild-type ion channels in live cells. Proc Natl Acad Sci U S A. 2014;111:E4789–E4796. doi: 10.1073/pnas.1406876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bingham JP, Bian S, Tan ZY, Takacs Z, Moczydlowski E. Synthesis of a biotin derivative of iberiotoxin: binding interactions with streptavidin and the BK Ca2+-activated K+ channel expressed in a human cell line. Bioconjug Chem. 2006;17:689–699. doi: 10.1021/bc060002u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hafidi A, Beurg M, Dulon D. Localization and developmental expression of BK channels in mammalian cochlear hair cells. Neuroscience. 2005;130:475–484. doi: 10.1016/j.neuroscience.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 74.Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 75.Abiraman K, Sah M, Walikonis RS, Lykotrafitis G, Tzingounis AV. Tonic PKA activity regulates SK channel nanoclustering and somatodendritic distribution. J Mol Biol. 2016;428:2521–2537. doi: 10.1016/j.jmb.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 76.Abiraman K, Tzingounis AV, Lykotrafitis G. K(Ca)2 channel localization and regulation in the axon initial segment. FASEB J. 2018;32:1794–1805. doi: 10.1096/fj.201700605R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takemoto Y, Slough DP, Meinke G, et al. Structural basis for the antiarrhythmic blockade of a potassium channel with a small molecule. FASEB J. 2018;32:1778–1793. doi: 10.1096/fj.201700349R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muroski ME, Oh E, Deschamps JR, Delehanty JB (2019) Gold nanoparticle bioconjugates: display of potassium channel–blocking tertiapin-Q peptides on gold nanoparticles enhances depolarization of cellular membrane potential. Part Part Syst Char 36:1970007
- 79.Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mantegazza M, Cestele S, Catterall WA. Sodium channelopathies of skeletal muscle and brain. Physiol Rev. 2021;101:1633–1689. doi: 10.1152/physrev.00025.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gilchrist J, Olivera BM, Bosmans F. Animal toxins influence voltage-gated sodium channel function. Handb Exp Pharmacol. 2014;221:203–229. doi: 10.1007/978-3-642-41588-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dutertre S, Lewis RJ. Use of venom peptides to probe ion channel structure and function. J Biol Chem. 2010;285:13315–13320. doi: 10.1074/jbc.R109.076596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stevens M, Peigneur S, Tytgat J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front Pharmacol. 2011;2:71. doi: 10.3389/fphar.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agnew WS, Moore AC, Levinson SR, Raftery MA. Identification of a large molecular weight peptide associated with a tetrodotoxin binding protein from the electroplax of Electrophorus electricus. Biochem Biophys Res Commun. 1980;92:860–866. doi: 10.1016/0006-291X(80)90782-2. [DOI] [PubMed] [Google Scholar]
- 85.Noda M, Suzuki H, Numa S, Stuhmer W. A single point mutation confers tetrodotoxin and saxitoxin insensitivity on the sodium channel II. FEBS Lett. 1989;259:213–216. doi: 10.1016/0014-5793(89)81531-5. [DOI] [PubMed] [Google Scholar]
- 86.Hille B. Ion channels of excitable membranes. 3. Sunderland, Mass.: Sinauer; 2001. [Google Scholar]
- 87.Angelides KJ, Nutter TJ. Molecular and cellular mapping of the voltage-dependent na channel. Biophys J. 1984;45:31–34. doi: 10.1016/S0006-3495(84)84096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Angelides KJ. Fluorescently labelled Na+ channels are localized and immobilized to synapses of innervated muscle fibres. Nature. 1986;321:63–66. doi: 10.1038/321063a0. [DOI] [PubMed] [Google Scholar]
- 89.Angelides KJ, Elmer LW, Loftus D, Elson E. Distribution and lateral mobility of voltage-dependent sodium channels in neurons. J Cell Biol. 1988;106:1911–1925. doi: 10.1083/jcb.106.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Joe EH, Angelides KJ. Clustering and mobility of voltage-dependent sodium channels during myelination. J Neurosci. 1993;13:2993–3005. doi: 10.1523/JNEUROSCI.13-07-02993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Massensini AR, Suckling J, Brammer MJ, Moraes-Santos T, Gomez MV, Romano-Silva MA. Tracking sodium channels in live cells: confocal imaging using fluorescently labeled toxins. J Neurosci Methods. 2002;116:189–196. doi: 10.1016/S0165-0270(02)00040-7. [DOI] [PubMed] [Google Scholar]
- 92.Ondrus AE, Lee HL, Iwanaga S, et al. Fluorescent saxitoxins for live cell imaging of single voltage-gated sodium ion channels beyond the optical diffraction limit. Chem Biol. 2012;19:902–912. doi: 10.1016/j.chembiol.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Montnach J, De Waard S, Nicolas S, et al. Fluorescent- and tagged-protoxin II peptides: potent markers of the Na(v) 1.7 channel pain target. Br J Pharmacol. 2021;178:2632–2650. doi: 10.1111/bph.15453. [DOI] [PubMed] [Google Scholar]
- 94.Gonzales J, de Souza D, Franca P, Jiang Y, et al. Fluorescence imaging of peripheral nerves by a Na(v)1.7-targeted inhibitor cystine knot peptide. Bioconjug Chem. 2019;30:2879–2888. doi: 10.1021/acs.bioconjchem.9b00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gonzales J, Pirovano G, Chow CY, et al. Fluorescence labeling of a Na(V)1.7-targeted peptide for near-infrared nerve visualization. EJNMMI Res. 2020;10:49. doi: 10.1186/s13550-020-00630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Catterall WA, Lenaeus MJ, Gamal El-Din TM. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu Rev Pharmacol Toxicol. 2020;60:133–154. doi: 10.1146/annurev-pharmtox-010818-021757. [DOI] [PubMed] [Google Scholar]
- 97.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 98.Bourinet E, Zamponi GW. Block of voltage-gated calcium channels by peptide toxins. Neuropharmacology. 2017;127:109–115. doi: 10.1016/j.neuropharm.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 99.Llinas R, Sugimori M, Lin JW, Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989;86:1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-Z. [DOI] [PubMed] [Google Scholar]
- 101.Ovsepian SV, Friel DD. The leaner P/Q-type calcium channel mutation renders cerebellar Purkinje neurons hyper-excitable and eliminates Ca2+-Na+ spike bursts. Eur J Neurosci. 2008;27:93–103. doi: 10.1111/j.1460-9568.2007.05998.x. [DOI] [PubMed] [Google Scholar]
- 102.Nakanishi S, Fujii A, Kimura T, Sakakibara S, Mikoshiba K. Spatial distribution of omega-agatoxin IVA binding sites in mouse brain slices. J Neurosci Res. 1995;41:532–539. doi: 10.1002/jnr.490410413. [DOI] [PubMed] [Google Scholar]
- 103.Nakanishi S. A spider toxin, omega-agatoxin IV A, binds to fixed as well as living tissues: cytochemical visualization of P/Q-type calcium channels. Microscopy (Oxf) 2016;65:337–340. doi: 10.1093/jmicro/dfw013. [DOI] [PubMed] [Google Scholar]
- 104.Jones OT, Kunze DL, Angelides KJ. Localization and mobility of omega-conotoxin-sensitive Ca2+ channels in hippocampal CA1 neurons. Science. 1989;244:1189–1193. doi: 10.1126/science.2543080. [DOI] [PubMed] [Google Scholar]
- 105.Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-E. [DOI] [PubMed] [Google Scholar]
- 106.Cohen MW, Jones OT, Angelides KJ. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J Neurosci. 1991;11:1032–1039. doi: 10.1523/JNEUROSCI.11-04-01032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Komuro H, Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992;257:806–809. doi: 10.1126/science.1323145. [DOI] [PubMed] [Google Scholar]
- 108.Mills LR, Niesen CE, So AP, Carlen PL, Spigelman I, Jones OT. N-type Ca2+ channels are located on somata, dendrites, and a subpopulation of dendritic spines on live hippocampal pyramidal neurons. J Neurosci. 1994;14:6815–6824. doi: 10.1523/JNEUROSCI.14-11-06815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones OT, Bernstein GM, Jones EJ, et al. N-Type calcium channels in the developing rat hippocampus: subunit, complex, and regional expression. J Neurosci. 1997;17:6152–6164. doi: 10.1523/JNEUROSCI.17-16-06152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bernstein GM, Mendonca A, Wadia J, Burnham WM, Jones OT. Kindling induces a long-term enhancement in the density of N-type calcium channels in the rat hippocampus. Neuroscience. 1999;94:1083–1095. doi: 10.1016/S0306-4522(99)00371-1. [DOI] [PubMed] [Google Scholar]
- 111.Kim HJ, Lee PC, Hong JH. Chloride channels and transporters: roles beyond classical cellular homeostatic pH or ion balance in cancers. Cancers (Basel) 2022;14:856. doi: 10.3390/cancers14040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gururaja Rao S, Patel NJ, Singh H. Intracellular chloride channels: novel biomarkers in diseases. Front Physiol. 2020;11:96. doi: 10.3389/fphys.2020.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 114.Jentsch TJ, Pusch M. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol Rev. 2018;98:1493–1590. doi: 10.1152/physrev.00047.2017. [DOI] [PubMed] [Google Scholar]
- 115.Stauber T, Weinert S, Jentsch TJ. Cell biology and physiology of CLC chloride channels and transporters. Compr Physiol. 2012;2:1701–1744. doi: 10.1002/cphy.c110038. [DOI] [PubMed] [Google Scholar]
- 116.Bordon KCF, Cologna CT, Fornari-Baldo EC, et al. From animal poisons and venoms to medicines: achievements, challenges and perspectives in drug discovery. Front Pharmacol. 2020;11:1132. doi: 10.3389/fphar.2020.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Phys. 1993;264:C361–C369. doi: 10.1152/ajpcell.1993.264.2.C361. [DOI] [PubMed] [Google Scholar]
- 118.Cohen G, Burks SR, Frank JA (2018) Chlorotoxin-a multimodal imaging platform for targeting glioma tumors. Toxins (Basel) 10(12):496. 10.3390/toxins10120496 [DOI] [PMC free article] [PubMed]
- 119.Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278:4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- 120.Duenas-Cuellar RA, Santana CJC, Magalhaes ACM, Pires OR, Jr, Fontes W, Castro MS. Scorpion toxins and ion channels: potential applications in cancer therapy. Toxins (Basel) 2020;12:326. doi: 10.3390/toxins12050326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Veiseh M, Gabikian P, Bahrami SB, et al. Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 122.Akcan M, Stroud MR, Hansen SJ, et al. Chemical re-engineering of chlorotoxin improves bioconjugation properties for tumor imaging and targeted therapy. J Med Chem. 2011;54:782–787. doi: 10.1021/jm101018r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Butte PV, Mamelak A, Parrish-Novak J, et al. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg Focus. 2014;36:E1. doi: 10.3171/2013.11.FOCUS13497. [DOI] [PubMed] [Google Scholar]
- 124.AV DS, Lin H, Henderson ER, Samkoe KS, Pogue BW. Review of fluorescence guided surgery systems: identification of key performance capabilities beyond indocyanine green imaging. J Biomed Opt. 2016;21:80901. doi: 10.1117/1.JBO.21.8.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fidel J, Kennedy KC, Dernell WS, et al. Preclinical validation of the utility of BLZ-100 in providing fluorescence contrast for imaging spontaneous solid tumors. Cancer Res. 2015;75:4283–4291. doi: 10.1158/0008-5472.CAN-15-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baik FM, Hansen S, Knoblaugh SE, et al. Fluorescence identification of head and neck squamous cell carcinoma and high-risk oral dysplasia with BLZ-100, a chlorotoxin-indocyanine green conjugate. JAMA Otolaryngol Head Neck Surg. 2016;142:330–338. doi: 10.1001/jamaoto.2015.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamada M, Miller DM, Lowe M, et al. A first-in-human study of BLZ-100 (tozuleristide) demonstrates tolerability and safety in skin cancer patients. Contemp Clin Trials Commun. 2021;23:100830. doi: 10.1016/j.conctc.2021.100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kovar JL, Curtis E, Othman SF, Simpson MA, Olive DM. Characterization of IRDye 800CW chlorotoxin as a targeting agent for brain tumors. Anal Biochem. 2013;440:212–219. doi: 10.1016/j.ab.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 129.Veiseh O, Sun C, Fang C, et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009;69:6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meng XX, Wan JQ, Jing M, Zhao SG, Cai W, Liu EZ. Specific targeting of gliomas with multifunctional superparamagnetic iron oxide nanoparticle optical and magnetic resonance imaging contrast agents. Acta Pharmacol Sin. 2007;28:2019–2026. doi: 10.1111/j.1745-7254.2007.00661.x. [DOI] [PubMed] [Google Scholar]
- 131.Yu XF, Sun Z, Li M, et al. Neurotoxin-conjugated upconversion nanoprobes for direct visualization of tumors under near-infrared irradiation. Biomaterials. 2010;31:8724–8731. doi: 10.1016/j.biomaterials.2010.07.099. [DOI] [PubMed] [Google Scholar]
- 132.Xiang Y, Liang L, Wang X, Wang J, Zhang X, Zhang Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicine-loaded liposomes. J Control Release. 2011;152:402–410. doi: 10.1016/j.jconrel.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 133.Qin C, He B, Dai W, et al. Inhibition of metastatic tumor growth and metastasis via targeting metastatic breast cancer by chlorotoxin-modified liposomes. Mol Pharm. 2014;11:3233–3241. doi: 10.1021/mp400691z. [DOI] [PubMed] [Google Scholar]
- 134.Chen S, Ahmadiantehrani M, Publicover NG, Hunter KW, Jr, Zhu X. Thermal decomposition based synthesis of Ag-In-S/ZnS quantum dots and their chlorotoxin-modified micelles for brain tumor cell targeting. RSC Adv. 2015;74:60612–60620. doi: 10.1039/C5RA11250H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Agarwal S, Mohamed MS, Mizuki T, Maekawa T, Sakthi Kumar D. Chlorotoxin modified morusin-PLGA nanoparticles for targeted glioblastoma therapy. J Mater Chem B. 2019;7:5896–5919. doi: 10.1039/C9TB01131E. [DOI] [PubMed] [Google Scholar]
- 136.Patil R, Galstyan A, Sun T, et al. Polymalic acid chlorotoxin nanoconjugate for near-infrared fluorescence guided resection of glioblastoma multiforme. Biomaterials. 2019;206:146–159. doi: 10.1016/j.biomaterials.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu C, Hansen SJ, Hou Q, et al. Design of highly emissive polymer dot bioconjugates for in vivo tumor targeting. Angew Chem Int Ed Eng. 2011;50:3430–3434. doi: 10.1002/anie.201007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ullrich N, Sontheimer H. Biophysical and pharmacological characterization of chloride currents in human astrocytoma cells. Am J Phys. 1996;270:C1511–C1521. doi: 10.1152/ajpcell.1996.270.5.C1511. [DOI] [PubMed] [Google Scholar]
- 139.Ullrich N, Bordey A, Gillespie GY, Sontheimer H. Expression of voltage-activated chloride currents in acute slices of human gliomas. Neuroscience. 1998;83:1161–1173. doi: 10.1016/S0306-4522(97)00456-9. [DOI] [PubMed] [Google Scholar]
- 140.Zeng XC, Li WX, Zhu SY, et al. Cloning and characterization of a cDNA sequence encoding the precursor of a chlorotoxin-like peptide from the Chinese scorpion Buthus martensii Karsch. Toxicon. 2000;38:1009–1014. doi: 10.1016/S0041-0101(99)00212-3. [DOI] [PubMed] [Google Scholar]
- 141.Fu YJ, Yin LT, Wang W, Chai BF, Liang AH. Synthesis, expression and purification of a type of chlorotoxin-like peptide from the scorpion, Buthus martensii Karsch, and its acute toxicity analysis. Biotechnol Lett. 2005;27:1597–1603. doi: 10.1007/s10529-005-2514-2. [DOI] [PubMed] [Google Scholar]
- 142.Fan S, Sun Z, Jiang D, et al. BmKCT toxin inhibits glioma proliferation and tumor metastasis. Cancer Lett. 2010;291:158–166. doi: 10.1016/j.canlet.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 143.Fu Y, An N, Zheng S, Liang A, Li Y. BmK CT-conjugated fluorescence nanodiamond as potential glioma-targeted imaging and drug. Diam Relat Mater. 2012;21:73–76. doi: 10.1016/j.diamond.2011.10.010. [DOI] [Google Scholar]
- 144.Zhao Y, McVeigh BM, Moiseenkova-Bell VY. Structural pharmacology of TRP channels. J Mol Biol. 2021;433:166914. doi: 10.1016/j.jmb.2021.166914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Goor MKC, Hoenderop JGJ, van der Wijst J. TRP channels in calcium homeostasis: from hormonal control to structure-function relationship of TRPV5 and TRPV6. Biochim Biophys Acta, Mol Cell Res. 2017;1864:883–893. doi: 10.1016/j.bbamcr.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 146.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 147.Minke B. TRP channels and Ca2+ signaling. Cell Calcium. 2006;40:261–275. doi: 10.1016/j.ceca.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 149.Meng J, Ovsepian SV, Wang J, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009;29:4981–4992. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O'Leary VB, O'Connell M, Antyborzec I, Ntziachristos V, Oliver Dolly J, Ovsepian SV. Alleviation of trigeminal nociception using p75 neurotrophin receptor targeted lentiviral interference therapy. Neurotherapeutics. 2018;15:489–499. doi: 10.1007/s13311-018-0608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bae C, Kalia J, Song I, et al. High yield production and refolding of the double-knot toxin, an activator of TRPV1 channels. PLoS One. 2012;7:e51516. doi: 10.1371/journal.pone.0051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sarkar D, Mishra S, Nisal R, et al. Site-specific fluorescent labeling of the cysteine-rich toxin, DkTx, for TRPV1 ion channel imaging and membrane binding studies. Bioconjug Chem. 2022;33:1761–1770. doi: 10.1021/acs.bioconjchem.2c00355. [DOI] [PubMed] [Google Scholar]
- 153.Lehen'kyi V, Raphael M, Prevarskaya N. The role of the TRPV6 channel in cancer. J Physiol. 2012;590:1369–1376. doi: 10.1113/jphysiol.2011.225862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stewart JM. TRPV6 as a target for cancer therapy. J Cancer. 2020;11:374–387. doi: 10.7150/jca.31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wissenbach U, Niemeyer B, Himmerkus N, Fixemer T, Bonkhoff H, Flockerzi V. TRPV6 and prostate cancer: cancer growth beyond the prostate correlates with increased TRPV6 Ca2+ channel expression. Biochem Biophys Res Commun. 2004;322:1359–1363. doi: 10.1016/j.bbrc.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 156.Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–7861. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- 157.Bowen CV, DeBay D, Ewart HS, et al. In vivo detection of human TRPV6-rich tumors with anti-cancer peptides derived from soricidin. PLoS One. 2013;8:e58866. doi: 10.1371/journal.pone.0058866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bychkov ML, Kirichenko AV, Shulepko MA, Mikhaylova IN, Kirpichnikov MP, Lyukmanova EN. Mambalgin-2 inhibits growth, migration, and invasion of metastatic melanoma cells by targeting the channels containing an ASIC1a subunit whose up-regulation correlates with poor survival prognosis. Biomedicines. 2021;9:1324. doi: 10.3390/biomedicines9101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee W, Leddy HA, Chen Y, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A. 2014;111:E5114–E5122. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu J, Goyal R, Grandl J. Localized force application reveals mechanically sensitive domains of Piezo1. Nat Commun. 2016;7:12939. doi: 10.1038/ncomms12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Diochot S, Baron A, Salinas M, et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature. 2012;490:552–555. doi: 10.1038/nature11494. [DOI] [PubMed] [Google Scholar]
- 162.Kapoor N, Lee W, Clark E, et al. Interaction of ASIC1 and ENaC subunits in human glioma cells and rat astrocytes. Am J Phys Cell Phys. 2011;300:C1246–C1259. doi: 10.1152/ajpcell.00199.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moore JW, Narahashi T, Ulbricht W. Sodium conductance shift in an axon internally perfused with a sucrose and low-potassium solution. J Physiol. 1964;172:163–173. doi: 10.1113/jphysiol.1964.sp007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Narahashi T, Moore JW, Scott WR. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J Gen Physiol. 1964;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kalia J, Milescu M, Salvatierra J, et al. From foe to friend: using animal toxins to investigate ion channel function. J Mol Biol. 2015;427:158–175. doi: 10.1016/j.jmb.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dolly JO, Parcej DN. Molecular properties of voltage-gated K+ channels. J Bioenerg Biomembr. 1996;28:231–253. doi: 10.1007/BF02110698. [DOI] [PubMed] [Google Scholar]
- 167.Dolly JO, Lawrence GW, Meng J, Wang J, Ovsepian SV. Neuro-exocytosis: botulinum toxins as inhibitory probes and versatile therapeutics. Curr Opin Pharmacol. 2009;9:326–335. doi: 10.1016/j.coph.2009.03.004. [DOI] [PubMed] [Google Scholar]