Fig. 1 |. Cellular localization and organization of neuronal spectrins.
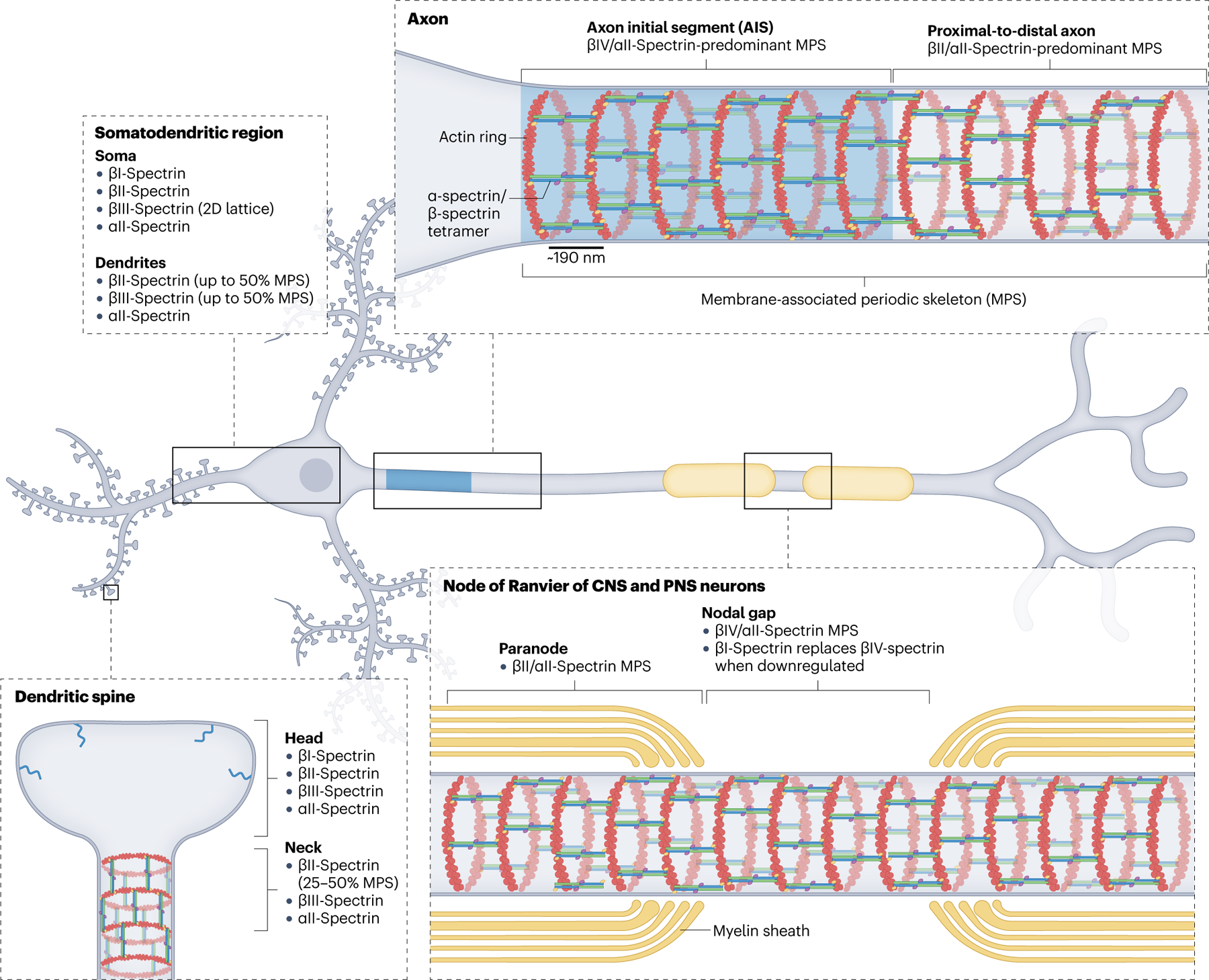
Mammalian neurons express six of the seven spectrins, which follow a general pattern of domain localization and organization across different neuron types. βIV-spectrin is enriched at the axon initial segment (AIS), where, together with αII-spectrin, actin and other key molecules, it forms a membrane-associated periodic skeleton (MPS)34. The MPS is best characterized by actin rings enwrapping the circumference of neuronal processes with a ~190 nm periodicity, which is determined by their cross-linking by spectrin tetramers in their fully elongated conformation. The proximal-to-distal axon, including axonal branches, expresses βII-spectrin and αII-spectrin in high abundance together with relatively less abundant βIII-spectrin, all integrated into the MPS that spans the full axon. In myelinated axons of both the CNS and the PNS, βIV/αII-spectrins are localized and periodically organized in the nodal gap of nodes of Ranvier (NoR), flanked by βII/αII-spectrins in the paranode, also periodically distributed60. Upon loss of βIV-spectrin, βI-spectrin localizes to NoR and rescues βIV-spectrin function. This redundancy is not available at the AIS, probably because βI-spectrin localization depends on its molecular partner ankyrin-R, which is not recruited to the AIS59. Unlike in the axon, the probability of detecting the MPS in dendritic shafts of mature neurons, which includes βII-spectrin and βIII-spectrin, is about 50%33. In addition to the quasi-1D organization of the MPS, βIII-spectrin can form 2D polygonal lattices in the soma and dendritic shaft. In dendritic spines, βII-spectrin and βIII-spectrin adopt MPS periodicity in the neck, but not in the head.
