Fig. 2 |. Tetrameric assembly and structural domains of neuronal spectrins.
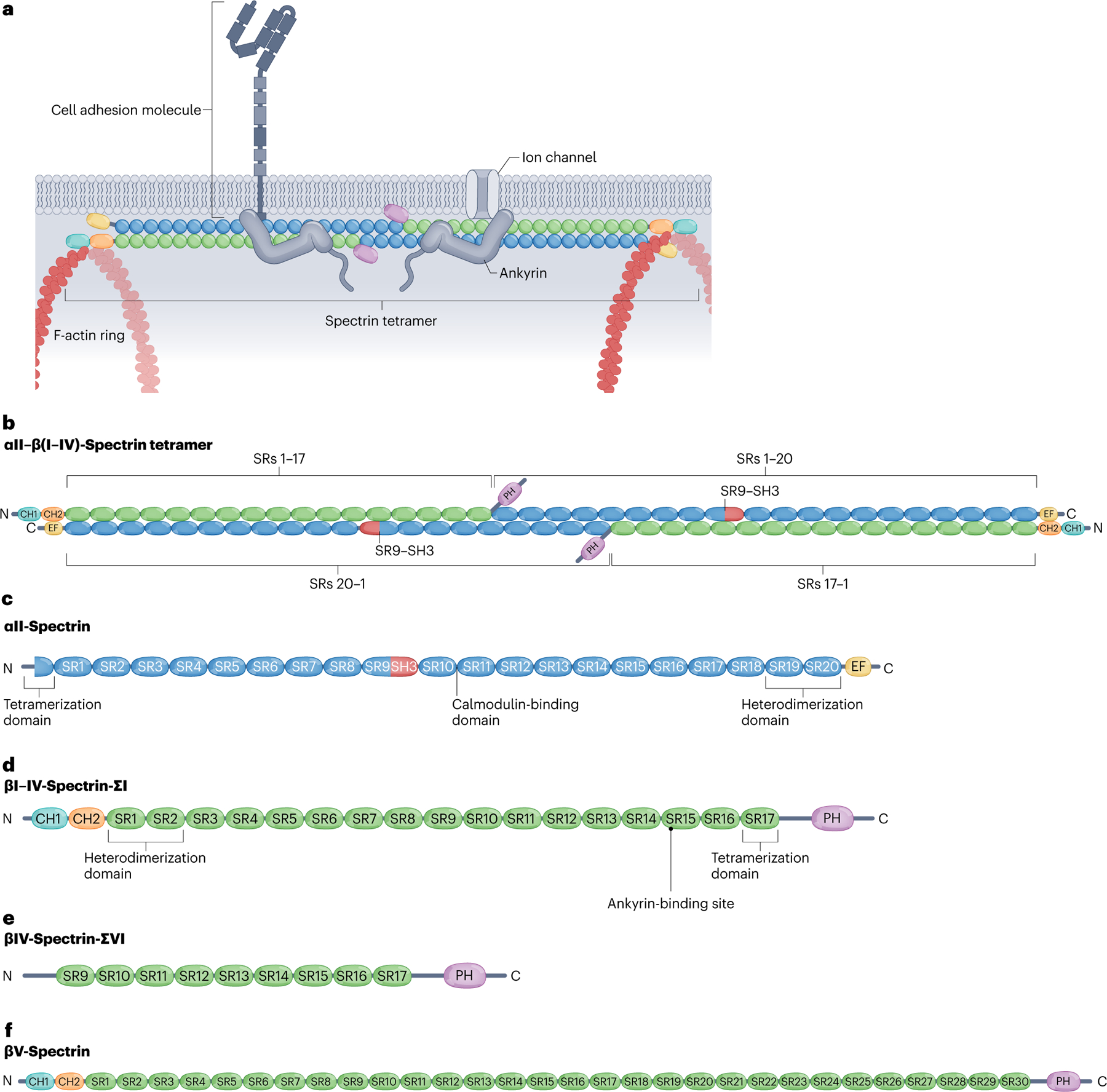
a, Canonical spectrins form heterotetramers of two α-units and two β-units that crosslink F-actin rings along the neuronal membrane. Spectrins bind ankyrins, which in turn stabilize membrane-spanning proteins such as cell adhesion molecules and ion channels. b, Spectrin tetramers assemble by linking heterodimers head-to-head via non-covalent association between the partial spectrin repeats (SRs) in the N terminus adjacent to SR1 in the α-spectrin subunits (blue) and partial SR17 at the N terminus of the β-spectrin subunits (green). Complementary motifs in SR1 and SR2 of βI–IV spectrins and SR19 and SR20 of αII-spectrin bind covalently to enable the antiparallel lateral assembly of α–β-spectrin heterodimers. c, αII-Spectrin spans 20 modular SRs (blue), a calcium-binding EF hand domain (yellow) close to the C terminus, an Src-homology 3 (SH3) domain (red) in SR9 and a calmodulin (CaM)-binding loop in SR10. d, Canonical βI–βIV-spectrins contain 16 full SRs and a partial 17th SR (green), two N-terminal tandem calponin homology (CH) domains (teal and orange), an ankyrin-binding site in SR15 and a C-terminal pleckstrin homology (PH) domain (purple). The CH domains enable binding to actin and the PH domain binds membrane lipids. e, The alternatively spliced βIV-spectrin-ΣVI isoform, which is important for maintenance of the axon initial segment (AIS), lacks the CH domains and the first eight full SRs, but retains ankyrin-binding activity. f, Giant βV-spectrin contains 29 full SRs plus a partial 30th SR. Whether βV-spectrin associates with αII-spectrin is not clear.
