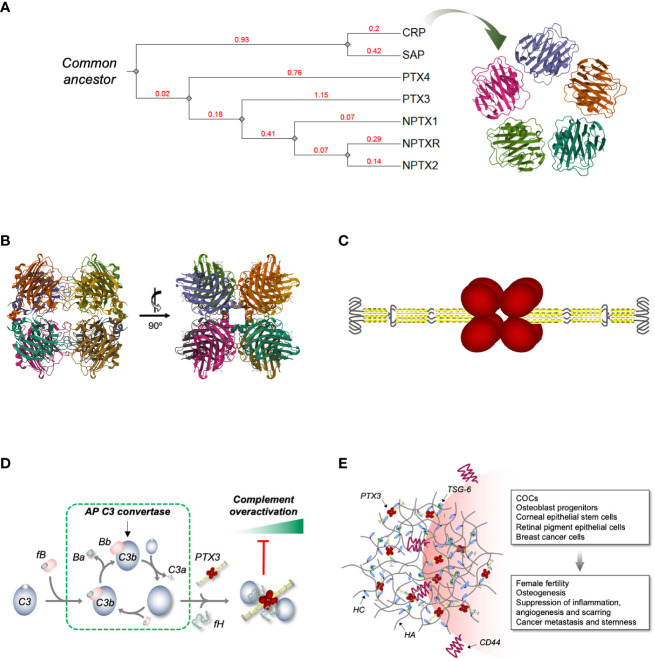
Figure 1.
Phylogenetic relationships across human pentraxins, 3D model of PTX3 and its interplay with complement and HA ECM. (A) Phylogenetic tree of the human PTX family of proteins. The tree was constructed using the Phylogeny.fr web server [http://www.phylogeny.fr/ and (6)], edited and annotated with the iTOL (interactive Tree Of Life) online tool [https://itol.embl.de/ and (7)]. The extent of genetic variation across members of the family (i.e., the average number of amino acid substitutions per site) is indicated by the branch lengths. These are drawn to scale and expressed as arbitrary unit (in red) with tree nodes represented by grey diamonds. The accession numbers used for the analysis are NP_000558.2 (CRP), NP_001630.1 (SAP), NP_002843.2 (PTX3), NP_001013680.1 (PTX4), NP_002513.2 (NPTX1), NP_002514.1 (NPTX2) and NP_055108.2 (NPTXR). Also, a 3D model of human CRP is shown [PDB-ID: 1GNH and (8)] that highlights the symmetric pentameric quaternary structure of this short pentraxin. (B) Orthogonal views of a high-resolution model of the C-terminal pentraxin domains of the human PTX3 based on Cryo-EM that show these domains to fold into octamers with D4 symmetry [PDB-ID: 7ZL1 and (9)]. (C) Schematic drawing of the PTX3 protein with the N-terminal regions (in yellow) forming two parallel tetrameric coiled coils at the opposite sides of the C-terminal core (in red) [based on (9)]. Hinge and intrinsically disordered regions (represented by black lines) bring flexibility to the structure. (D) We have reported that surface bound PTX3 forms a ternary complex with fH and C3b that acts as a “hot spot” for AP inhibition (10). Indeed, when bound to PTX3 and fH, C3b is no longer accessible to factor B (fB, which during AP activation is proteolytically processed to Ba and Bb, the latter being a component of the AP C3 convertase) and loses the ability to amplify the complement cascade (with further cleavage of C3 to C3a and C3b) and the associated inflammatory response (including production of the anaphylatoxin C3a). (E) PTX3 is an essential component of the HA-rich ECMs that transiently form in inflammatory and inflammatory-like conditions. Incorporation of PTX3 in these matrices requires synthesis of covalent adducts between HA and heavy chains (HCs) of the proteoglycan IαI (HC•HA), a reaction that is catalyzed by the hyaladherin TSG-6. PTX3 makes multiple, non-covalent interactions with the HC components of the HC•HA complex, and in this way cross-links HA. This mechanism has major implications in female fertility (11) and has been associated with the anti-inflammatory, -angiogenic and -scarring properties of the PTX3/HC•HA complex isolated from the human amniotic membrane (12). Also, through the HA receptor CD44, HA-embedded PTX3 has been recently reported to promote osteogenesis (13) and breast cancer growth, stemness and metastasis (14).