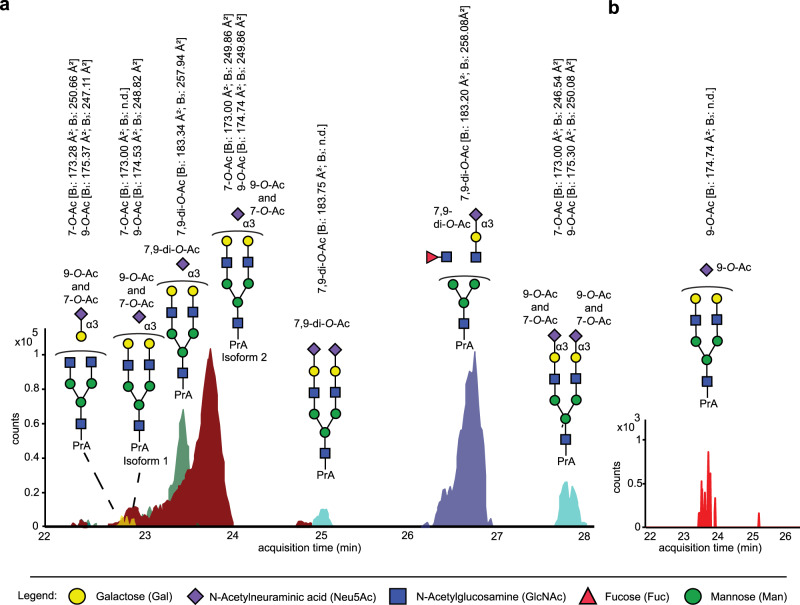
Fig. 3. O-acetylated (O-Ac) N-glycans derived from biologicals.
a Extracted ion chromatograms of glycans released from Myozyme and derivatized with procainamide (PrA) (n = 1) from left to right at m/z 925.8682 ([M + H + Na]2+, yellow), m/z 1006.8946 ([M + H + Na]2+, burgundy), m/z 1027.8999 ([M + H + Na]2+, green), m/z 1173.4476 ([M + H + Na]2+,cyan), m/z 1030.8934 m/z ([M + 2Na]2+, dark blue) and (b) Aflibercept (n = 1) (at m/z 1017.8856 ([M + 2Na]2+). The peaks were assigned to structures using accurate m/z values and sialic acid linkage and acetyl ester positions were determined by the CCS values of B1 and B3 ions; n.d. not determined.