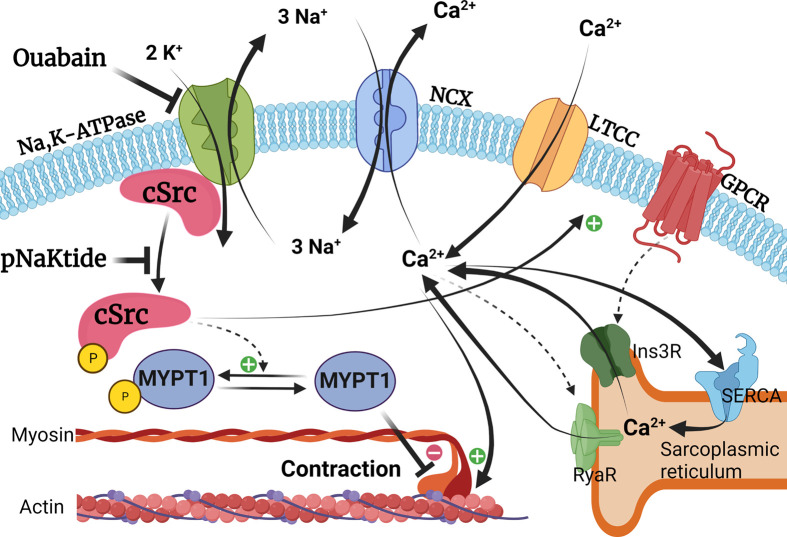
Figure 2. Signaling pathways behind potentiation of vascular smooth muscle contraction by ouabain.
The Na,K-ATPase generates the electrochemical gradient for Na+ ions, driving Ca2+ extrusion through the Na,Ca-exchanger (NCX) [45]. Upon binding of ouabain, the Na,K-ATPase pumping is impaired, reducing the NCX driving force and increasing intracellular Ca2+ concentration [43,46]. This surplus Ca2+ is then loaded into the sarcoplasmic reticulum by the sarcoendoplasmic reticulum Ca2+ ATPase (SERCA) [45,48]. Subsequently, more Ca2+ is released from the sarcoplasmic reticulum via the inositol trisphosphate receptors (Ins3R) upon G-protein coupled receptor (GPCR) stimulation and/or as a Ca2+-induced Ca2+ release through ryanodine receptors (RyaR) resulting in potentiated smooth muscle contraction [45,48]. Ouabain also induces cSrc kinase phosphorylation, ultimately increasing the sensitivity of the contractile machinery to intracellular Ca2+ [11,39]. This occurs through the phosphorylation of myosin phosphatase target subunit 1 (MYPT1), leading to the inhibition of the myosin light chain phosphatase, resulting in Ca2+ sensitization [10,11,39,49,50]. A synthetic peptide inhibitor, pNaKtide [51], inhibits cSrc kinase activation and thus, reduces smooth muscle Ca2+ sensitivity [11]. The cSrc kinase signaling may also control intracellular Ca2+ concentration through the phosphorylation of L-type calcium channels (LTCC), leading to increased Ca2+ influx [52,53].