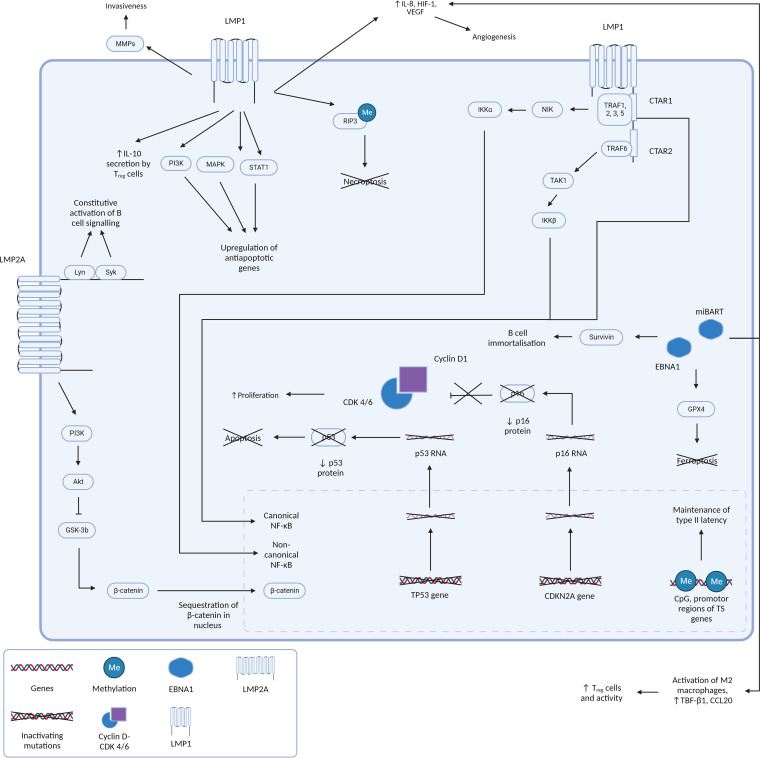
Figure 3.
Figure 3 presents a comprehensive and intricate depiction of the pivotal roles played by type II latency Epstein-Barr virus (EBV) oncoproteins, specifically Latent Membrane Proteins 1 and 2A (LMP1 and LMP2A), in conjunction with the crucial gene product EBNA1. It sheds light on how their orchestrated actions contribute to the initiation and progression of tumorigenesis by silencing of key tumor suppressor genes and driving cell proliferation and survival. LMP1 and LMP2A: masters of oncogenic signaling At the forefront of this dynamic interplay are the oncoproteins LMP1 and LMP2A. LMP1 is a multifunctional transmembrane protein. By emulating the constitutive activation of CD40, a pivotal B-cell receptor, LMP1 engages the TNF receptor-associated factors (TRAFs) and orchestrates the activation of the canonical NF-kB pathway. This activation culminates in the creation of a proinflammatory milieu that is conducive to cell survival and unbridled proliferation. Furthermore, LMP1’s capacity to activate the non-canonical NF-kB pathway further amplifies these signals, resulting in a sustained NF-kB activity that effectively shields cells from apoptotic cues. LMP1’s influence isn’t confined to NF-kB signaling alone. It interfaces with a multitude of molecules, including Janus kinases (JAKs), STATs, and PI3K, thereby activating pathways that foster cell growth and survival. Moreover, LMP1’s impact on various microRNAs and transcription factors significantly influences gene expression and cellular behavior. Beyond direct oncogenic signaling, LMP1 also modulates the tumor microenvironment, augment cellular invasiveness, and promotes angiogenesis. These help create a supportive niche for tumor progression and metastasis. Complementing LMP1, LMP2A emerges as a critical player in EBV-associated oncogenesis. This transmembrane protein mimics the B-cell receptor (BCR), a critical component in B-cell signaling. By recruiting protein tyrosine kinases, including Lyn and Syk, LMP2A effectively dampens BCR signaling. Activation of the PI3-kinase/Akt axis stimulates GSK3 which culminates in the accumulation of betacatenin, thereby promoting WNT signaling. This strategic inhibition aids in evading the regulatory mechanisms that would otherwise prompt apoptosis of autoreactive B cells. This evasion, in turn, promotes the survival of EBV-infected cells, and similar to LMP1, fosters a cellular milieu that supports enhanced proliferation and survival. EBNA1: guardian of viral persistence and cellular transformation Central to this network of interactions is the gene product EBNA1. This multifaceted protein stands as a linchpin in ensuring the persistence of EBV within the host cell. In addition to its role in maintaining viral genome replication, EBNA1 actively engages cellular machinery to promote cell proliferation and survival. Importantly, EBNA1 orchestrates the silencing of key tumor suppressor genes, notably CDKN2A and TP53. This results in an imbalance that shifts the cellular equilibrium toward unchecked cell growth and survival, fueling the malignant transformation of host cells. A holistic view of tumorigenesis Collectively, Figure 3 encapsulates the multifaceted orchestration of type II latency EBV oncoproteins, LMP1, LMP2A, and EBNA1, in driving the molecular intricacies of tumorigenesis. Their intricate molecular interplay, ranging from direct oncogenic signaling to the modulation of the tumor microenvironment, lays the foundation for cell transformation and the subsequent development of EBV-associated malignancies.