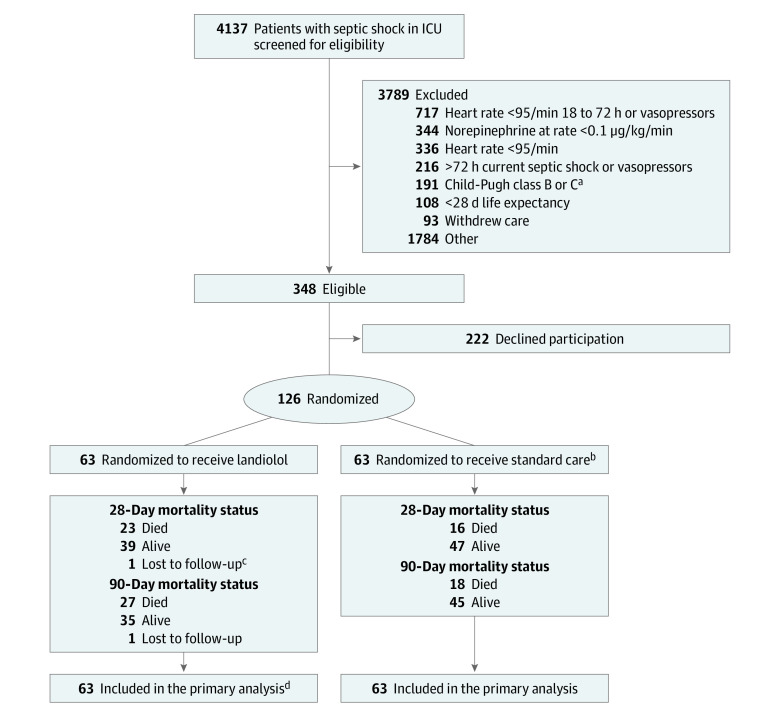
Figure 1. Flow of Patients in the STRESS-L Trial.
aA Child-Pugh score of 7 or more was excluded because of the risk of inappropriate inclusion in the study.
bOne patient was randomized in error because their heart rate was 84/min at the point of randomization. However, the patient remained in the trial on an intention-to-treat basis, and their routine data were collected.
cOnly 1 patient was recruited who had COVID-19.
dNine patients (7.1%) withdrew from treatment but remained in the follow-up. Of these, 8 were withdrawn by the clinician and 1 by their personal legal representative.