Figure 1.
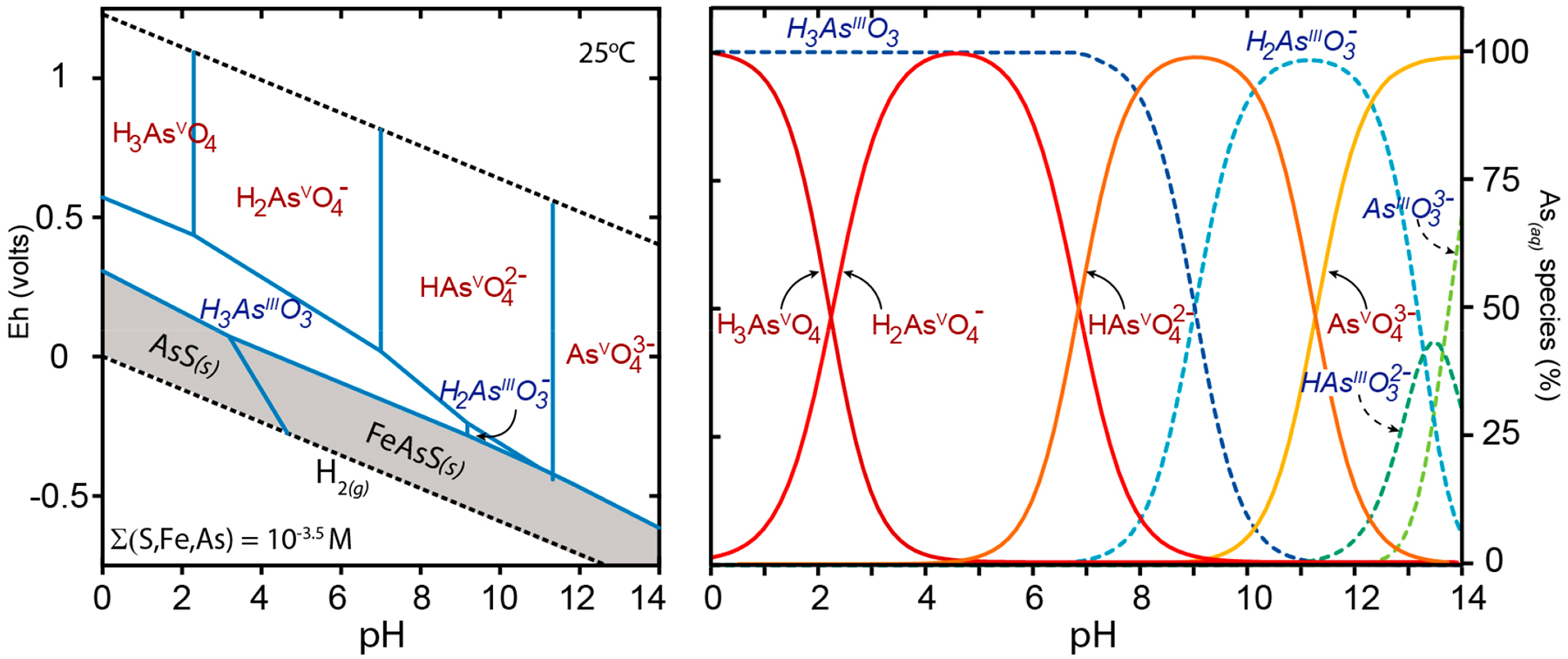
Eh-pH activity diagram of arsenic species at 25 °C, 1 bar, (S, Fe, As) = 10−3 M (left). Dashed lines bound the stability field of water, arsenate (AsV) species are shown in red, arsenite (AsIII) species are shown in blue italics, solid phases are shown with a darkened background. The distribution of pH-dependent dissolved arsenic species are shown (right) with arsenate as red solid lines and red text and arsenite in dashed blue lines with blue italic text. Dissolved arsenic species become protonated at low pH and the charge on the oxyanion decreases. Under environmental conditions (pH ≈ 5–9), arsenate generally exists as H2AsO4− and HAsO42−, while arsenite is the uncharged molecule H3AsO30. Under highly reducing conditions and in the presence of high sulfur activity, solid-phase arsenic sulfides (e.g., AsS) are stable.
