Abstract
We present a case of unilateral full-thickness macular hole (MH) successfully repaired with an amniotic membrane (AM) graft in a patient with Alport syndrome. A 58-year-old Asian female with past medical history of Alport syndrome diagnosed at early stage, presented with a 5-week history of vision loss in her right eye. Examination of her eyes showed normal retinal vessels and an MH measuring 1,300 μm in basal diameter, 806 μm in minimum linear diameter, and 490 μm in height in the right eye and macular thinning with laser scars inferiorly in the left eye. The patient underwent 23-g pars plana vitrectomy with intraocular lens explantation. After multiple unsuccessful attempts in inducing a posterior vitreous detachment around the optic nerve and in the posterior pole, a 1 mm AM graft placed on the MH and the edges tucked under the edges of the hole using a bimanual technique. Five months after surgery, the MH remained sealed with improved final vision. MHs are rare manifestations of Alport syndrome, and surgical treatment of Alport syndrome-associated MHs is challenging. However, further studies to explore new techniques using AM are needed.
Keywords: Alport syndrome, Macular hole, Amniotic membrane, Retina
Introduction
Alport syndrome is a rare hereditary condition characterized by sensorineural hearing loss, progressive renal failure, and variable ophthalmic abnormalities [1, 2]. The prevalence is 1–5,000 to 50,000 live births, with males more severely affected than females. Associated ophthalmic manifestations include corneal clouding, posterior polymorphous corneal dystrophy, cataract, anterior lenticonus, fleck retinopathy, foveal and mid-peripheral retinoschisis, and lamellar or full-thickness macular hole (MH). Surgical treatment of Alport syndrome-associated MHs is challenging [3, 4]. Alport syndrome is an inherited condition caused by mutations in COL4A3, COL4A4, or COL4A5 genes which affect the production of type IV collagen, manifesting in systemic abnormalities of the basement membrane. It is inherited in an x-linked fashion in most cases followed by autosomal recessive and rarely in autosomal dominant fashion [1, 2]. Alport syndrome is associated with multiple retinal findings, including dot and fleck retinopathy, macular thinning, peripheral inner retinal thinning, Bull’s eye maculopathy, and lamellar or full-thickness MH [4]. MHs are rare manifestations of Alport syndrome, and it is believed that they reflect the abnormal vitreoretinal interface, Bruch’s membrane, and internal limiting membrane (ILM) [3–5].
Herein, we report a case of unilateral full-thickness MH in a patient with Alport syndrome. To the best of our knowledge, this is the second reported case of repaired and sealed full-thickness MH with amniotic membrane (AM) graft in a patient with Alport syndrome.
Case Report
A 58-year-old Asian female presented with a 5-week history of vision loss in her right eye. Her past medical history included Alport syndrome diagnosed at early stage, hypertension, diabetes, hypoacusis, and end-stage renal disease requiring renal transplantation. Her past ocular history was significant for high myopia, peripheral laser for retinal holes in the left eye, cataract extraction with intraocular lens placement in both eyes (sulcus intraocular lens in OD, in the bag intraocular lens in OS), and subluxed intraocular lens of the right eye following a fall 2 years ago.
On examination, her best corrected visual acuity (BCVA) was 20/70 OD and 20/25 OS. Extraocular movements were intact, and pupils were round and reactive with no relative afferent pupil defect. The intraocular pressure (IOP) by applanation was 19 mm Hg OD and 18 mm Hg OS. Slit-lamp examination revealed a subluxed intraocular lens in the sulcus OD and a posterior chamber intraocular lens with mild posterior capsular opacity OS. Fundus examination of OD revealed no vitreous cells, cup-disc ratio of 0.45, normal retinal vessels, and an MH measuring 1,300 μm in basal diameter, 806 μm in minimum linear diameter, and 490 μm in height and macular thinning with laser scars inferiorly OS (Fig. 1).
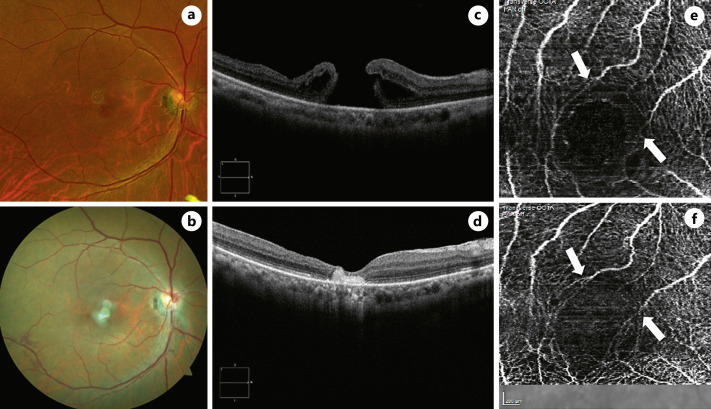
Fig. 1.
Color fundus photographs of large MH before surgery (a) and postoperative color fundus photo of sealed MH with AM graft (b). c Preoperative spectral domain optical coherence tomography (SD-OCT) shows a full-thickness MH measuring 1,300 μm in basal diameter, 806 μm in minimum linear diameter, and 490 μm in height with intraretinal fluid along edges. d SD-OCT 18 days after surgical repair shows hyperreflectivity corresponding to AM and confirms successful hole closure. Preoperative (e) and postoperative (f) OCT angiography appearance with vascular remodeling and foveal avascular zone with no significant abnormality in shape or size. The white arrows point to where the vascular remodeling took place.
The patient underwent 23-g pars plana vitrectomy with intraocular lens explantation. During surgery, multiple attempts at inducing a posterior vitreous detachment around the optic nerve and in the posterior pole were unsuccessful. Crystals of the dilute triamcinolone acetonide used to stain the vitreous were noted in the base of the MH, indicating that there was no vitreous above the MH. Brilliant Blue G did not stain the retinal surface. At this point, a 1 mm AM graft was harvested using a 1 mm biopsy punch, introduced in the vitreous cavity through one of the trocars, placed on the MH and the edges tucked under the edges of the hole using a bimanual technique. Air-fluid exchange was completed, and the vitreous cavity was filled with a 25% concentration of SF6. The patient was instructed to maintain a chin-down position. Her immediate postoperative course was complicated by elevated IOP that required multiple medications (simbrinza, timolol, and latanoprost), along with topical prednisolone.
A type 2B closure of the MH was confirmed by optical coherence tomography (OCT) 18 days post-surgery. Five months after surgery, the MH remained sealed with BCVA improvement to 20/40. IOP was normal off-drops. The patient opted for aphakic contact lens correction in the right eye. Figure 1 depicts the retinal imaging of both eyes pre- and postoperatively. Care checklist is presented as a supplementary material (for all online suppl. material, see https://doi.org/10.1159/000533712).
Discussion
Alport syndrome is a rare condition characterized by abnormal production of type IV collagen located in the ILM and Bruch’s membrane and is associated with several retinal findings, including thinning of the ILM and Bruch’s membrane [6]. Thinned ILM leads to increased vitreous traction susceptibility, and thinned Bruch’s membrane with increased permeability leads to intraretinal cyst formation. Multiple defects in the ILM followed by fluid passage through Bruch’s membrane and merging of the microcysts can lead to full-thickness MHs [6, 7]. Macular holes in Alport syndrome may also develop due to abnormal vitreomacular traction [8].
Full-thickness MHs are less common than lamellar holes among patients with Alport syndrome. When full-thickness MHs occur, they are often large and occur at a younger age [6, 9].
Since patients with Alport syndrome might lose their central vision from lenticonus, close follow-up with OCT to assess the retinal layers should be used to monitor the foveal status. In our patient, OCT angiography showed evidence of vascular remodeling around the fovea.
There are several cases of full-thickness MH and bilateral giant MH in patients with Alport syndrome where no intervention was offered to the patients [4, 8–10]. Vitrectomy, ILM peeling, and intraocular tamponade are the standard treatment options for MH repair, but in the case of thinned or missing ILM and adherent posterior hyaloid, such as in Alport patients, standard MH surgery can be unsuccessful [11–14]. The abnormal vitreoretinal interface and weakened basement membrane contribute to surgical intervention failure [6, 12].
MH surgery in patients with Alport syndrome has resulted in closure of hole with an improved vision, though the tight adherence of posterior hyaloid to the macula and several unsuccessful attempts for the removal of the posterior cortical vitreous have been reported [8, 11]. In a case of unilateral full-thickness MH and evidence of vitreofoveal traction in the fellow eye in a patient with Alport syndrome reported by Randhawa S. et al., 25-gauge pars plana vitrectomy, ILM peeling, and fluid gas exchange for the MH were performed. Since the posterior hyaloid was very adherent in that case, careful separation of the posterior hyaloid with an extrusion needle with high aspiration, and retinal forceps to peel the ILM beyond the vascular arcade has shown successful closure 1 month after the surgery with VA of 20/60 [7]. Sanjuan et al. [13] reported a case of refractory full-thickness MH in a patient with Alport syndrome treated with vitrectomy combined with autologous neurosensory retinal flap and achieved anatomical closure of the MH with BCVA improved from 20/200 to 20/80.
Successful closure of large, refractory, or recurrent MH where ILM is missing has been achieved with the use of AM grafts [15]. Because the AM graft should be placed under the edges of the MH, with the chorion/stromal side facing the RPEs, careful placement of AM can be challenging [16–20]. Table 1 summarizes the previous studies on the surgical management of MH in Alport patients. There is only one reported case of MH in a patient with Alport syndrome that was repaired with AM graft similar to our patient with improved final visual outcome [19].
Table 1.
Summary of the previous studies on the surgical management of MH in Alport patients
| Patient characteristics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| study | age | gender | inheritance | other ocular comorbidities | size | VA | PVD preop | surgical technique | details | closure | |
| Miller et al. [11] (2007) | 35 | Female | Autosomal recessive | High myopia | 1,350 μm OD | 20/400 OD | No | PPV C3F8 | PVD unable | Yes | |
| Posterior subcapsular cataract, anterior lenticonus | |||||||||||
| 2,050 OS | 20/800 OS | ||||||||||
| Randhawa et al. [7] (2013) | 30 | Female | Anterior lenticonus | NA (large, about half DD in photo) OD | 20/100 OD | No | PPV ILMP | Yes | |||
| 20/25 OS | |||||||||||
| No hole OS | |||||||||||
| Chaudhry et al. [14] (2021) | 65 | Male | No hole OD | 20/32 OD | No | PPV SF6 | ILM absent | No | |||
| 453 μm OS | 20/80 OS | ||||||||||
| Sanjuán et al. [13] (2021) | 35 | Male | Previous failed PPV ILMP | No hole OD | NA | Yes | PPV, autologous retinal flap, SO | Yes | |||
| 1,333 μm, second hole with 838 μm OS | |||||||||||
| 20/200 OS | |||||||||||
Conclusion
To the best of our knowledge, our report is the second case of repaired MH with AM graft. Further studies to explore AM clinical implications and enhanced surgical techniques are needed.
Patient perspective: our patient was satisfied with the results of surgery. Five months after surgery, the MH remained sealed with BCVA improvement to 20/40.
Statement of Ethics
This case report was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a HIPPA (Health Insurance Portability and Accountability Act)-compliant manner. Study has been granted an exemption from requiring ethics approval by the IRB of the University of Arkansas for Medical Sciences. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
Dr. Sayena Jabbehdari reviewed medical record, wrote original draft of case report, and revised intellectual content. Dr. Pedro Teletlbom contributed to the original draft and manuscript revision. Dr. David Warner and Dr. Sami Uwaydat critically revised the manuscript and intellectual content.
Funding Statement
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author. All authors attest that they meet the current ICMJE criteria for authorship.
Supplementary Material
References
- 1. Scassa C, Cupo G, Bruno M, Iervolino R, Scarinci F, Giusti C. Early lamellar macular hole in Alport syndrome: case report and review of the literature. Eur Rev Med Pharmacol Sci. 2012;16(1):122–5. [PubMed] [Google Scholar]
- 2. Kasi SK, Adam MK, Ehmann DS. Bilateral retinal problem in a patient with alport syndrome. JAMA Ophthalmol. 2017 Sep 1;135(9):995–6. 10.1001/jamaophthalmol.2017.0036. [DOI] [PubMed] [Google Scholar]
- 3. Fawzi AA, Lee NG, Eliott D, Song J, Stewart JM. Retinal findings in patients with Alport Syndrome: expanding the clinical spectrum. Br J Ophthalmol. 2009 Dec;93(12):1606–11. 10.1136/bjo.2009.158089. [DOI] [PubMed] [Google Scholar]
- 4. Thomas AS, Baynham JT, Flaxel CJ. Macular holes, vitelliform lesions, and midperipheral RETINOSCHISIS IN alport syndrome. Retin Cases Brief Rep. 2016;10(2):109–11. 10.1097/ICB.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 5. Blasi MA, Rinaldi R, Renieri A, Petrucci R, De Bernardo C, Bruttini M, et al. Dot-and-fleck retinopathy in Alport syndrome caused by a novel mutation in the COL4A5 gene. Am J Ophthalmol. 2000 Jul;130(1):130–1. 10.1016/s0002-9394(00)00466-9. [DOI] [PubMed] [Google Scholar]
- 6. Savige J, Liu J, DeBuc DC, Handa JT, Hageman GS, Wang YY, et al. Retinal basement membrane abnormalities and the retinopathy of Alport syndrome. Invest Ophthalmol Vis Sci. 2010 Mar;51(3):1621–7. 10.1167/iovs.08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Randhawa S, Fu AD, Lujan BJ, McDonald HR, Jumper JM. Autofluorescence and spectral domain OCT findings in Alport syndrome. Retin Cases Brief Rep. 2013 Fall;7(4):376–9. 10.1097/ICB.0b013e318296e174. [DOI] [PubMed] [Google Scholar]
- 8. Shah SN, Weinberg DV. Giant macular hole in Alport syndrome. Ophthalmic Genet. 2010 Jun;31(2):94–7. 10.3109/13816811003767128. [DOI] [PubMed] [Google Scholar]
- 9. Rahman W, Banerjee S. Giant macular hole in Alport syndrome. Can J Ophthalmol. 2007 Apr;42(2):314–5. 10.3129/can.j.ophthalmol.i07-020. [DOI] [PubMed] [Google Scholar]
- 10. Raimundo M, Fonseca C, Silva R, Figueira J. Bilateral giant macular holes: a rare manifestation of Alport syndrome. Eur J Ophthalmol. 2019 Jan;29(1):NP13–6. 10.1177/1120672118781232. [DOI] [PubMed] [Google Scholar]
- 11. Miller JJ, Rodriguez FJ, Smiddy WE, Rodriguez A. Macular hole surgery in alport syndrome. Retin Cases Brief Rep. 2007 Summer;1(3):153–5. 10.1097/01.ICB.0000279647.91700.b9. [DOI] [PubMed] [Google Scholar]
- 12. Gupta V, Kumar N. Bilateral macular holes: an unusual feature of alport syndrome. Retina. 2002 Aug;22(4):499–501. 10.1097/00006982-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 13. Sanjuán P, Samaan M, Nadal J. Autologous retina transplantation for treatment of refractory double full-thickness macular hole in Alport syndrome. Retin Cases Brief Rep. 2023;17(2):89–92. 10.1097/ICB.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 14. Chaudhry SG, Liew G, Fung AT. Missing internal limiting membrane during macular hole repair in alport syndrome. Case Rep Ophthalmol. 2021 May 3;12(2):320–3. 10.1159/000513420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rizzo S, Caporossi T, Tartaro R, Finocchio L, Franco F, Barca F, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019 Oct;39(Suppl 1):S95–103. 10.1097/IAE.0000000000002320. [DOI] [PubMed] [Google Scholar]
- 16. Qiao G, Xie L, Zou Q, He C, Zhang X, Tang Z, et al. The use of biological amniotic membranes in the treatment of recurrent macular holes. Sci Rep. 2022 Nov 4;12(1):18661. 10.1038/s41598-022-21754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Felfeli T, Corrin M, Papanikolaou J, Mandelcorn ED. Macular hole hydrodissection technique with human amniotic membrane for repair of large macular holes. Retin Cases Brief Rep. 2022 Jun 2. Publish Ahead of Print. 10.1097/ICB.0000000000001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lorenzi U, Mehech J, Caporossi T, Romano MR, De Fazio R, Parrat E, et al. ; ReMaHo Study Group . A retrospective, multicenter study on the management of macular holes without residual internal limiting membrane: the refractory macular hole (ReMaHo) study. Graefes Arch Clin Exp Ophthalmol. 2022 Dec;260(12):3837–45. 10.1007/s00417-022-05739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferreira MA, Maia A, Machado AJ, Ferreira REA, Hagemann LF, Júnior PHER, et al. Human amniotic membrane for the treatment of large and refractory macular holes: a retrospective, multicentric, interventional study. Int J Retina Vitreous. 2021 May 8;7(1):38. 10.1186/s40942-021-00308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baradad-Jurjo MC, Vela Segarra JI, Díaz-Cascajosa J, Vilimelis JC, Bassaganyas F. Intraretinal human amniotic membrane after macular hole repair. Retin Cases Brief Rep. 2022 Aug 23ublish Ahead of Print. 10.1097/ICB.0000000000001332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author. All authors attest that they meet the current ICMJE criteria for authorship.