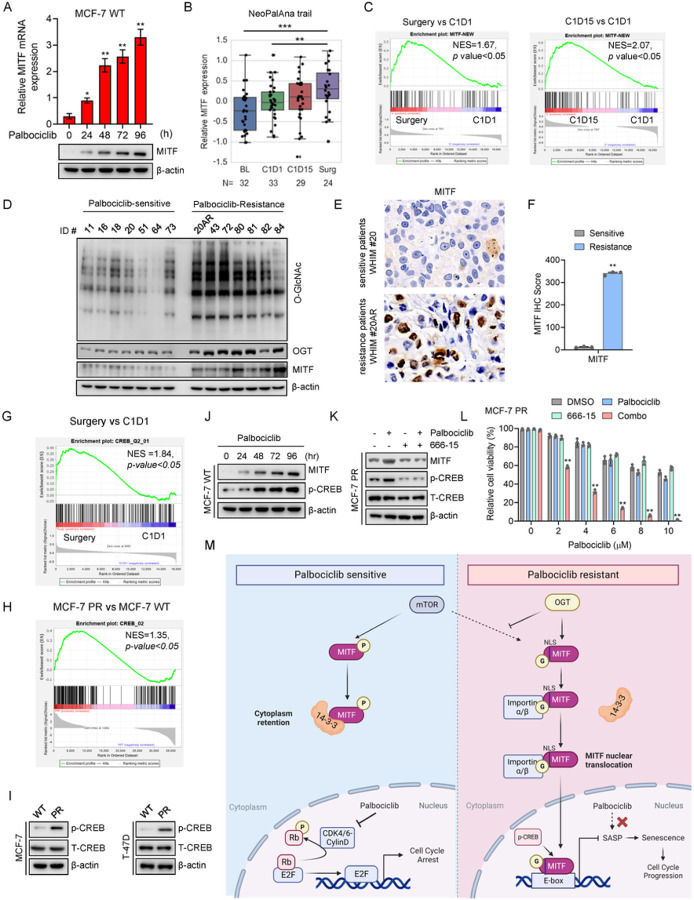
Figure 7. MITF-mediated pathway is activated in response to palbociclib and is elevated in tumors from palbociclib-resistant breast cancer patients.
(A) MCF-7 cells were collected after indicated treatments, and then subjected to qPCR and immunoblotting to examine expression levels of genes as indicated. (B) Expression of MITF in primary breast cancers from patients at stages of baseline (BL), 1 month after endocrine treatment (C1D1), 3 months after endocrine and palbociclib treatment (C1D15), and 12 weeks after endocrine and palbociclib treatment (Surgery) in the NeoPalAna trial (n represents the number of patient biopsies, noted in each graph). Data representing means ± SD of relative microarray reads are shown. (C) Left, GSEA profiling to show enrichment of the MITF signature geneset in the Surgery group vs. C1D1 group. Right, GSEA profiling to show the enrichment of the MITF signature geneset in the C1D15 group vs. C1D1 group. NES score and p values determined by GSEA software. (D) Palbociclib-resistant and sensitive breast cancer PDX lines were collected and subjected to immunoblotting for proteins as indicated. (E) Representative images of IHC staining for MITF proteins in PDX lines WHIM 20AR and WHIM 20. (F) Quantification of results of MITF and O-GlcNAc based on IHC score from E. (G) GSEA profiling to show the enrichment of the CREB geneset in the Surgery group vs. C1D1 group. (H) GSEA profiling to show the enrichment of CREB geneset in MCF-7 PR cells vs. MCF-7 cells. (I) MCF-7 and T-47D cells were collected and then subjected to immunoblotting for indicated proteins. (J-K) MCF-7 PR cells were collected and then subjected to immunoblotting for indicated proteins. (L) Cell viability was examined in MCF-7 PR cells treated with chemicals as indicated. Data represent means ± SD from three independent experiments. **, p£0.01. (M) Schematic of how O-GlcNAcylation of MITF contributes palbociclib resistance in breast cancer cells. See text for details.