Abstract
Introduction
Conventional interventional modalities for preserving or improving cognitive function in patients with brain tumour undergoing radiotherapy usually involve pharmacological and/or cognitive rehabilitation therapy administered at fixed doses or intensities, often resulting in suboptimal or no response, due to the dynamically evolving patient state over the course of disease. The personalisation of interventions may result in more effective results for this population. We have developed the CURATE.AI COR-Tx platform, which combines a previously validated, artificial intelligence-derived personalised dosing technology with digital cognitive training.
Methods and analysis
This is a prospective, single-centre, single-arm, mixed-methods feasibility clinical trial with the primary objective of testing the feasibility of the CURATE.AI COR-Tx platform intervention as both a digital intervention and digital diagnostic for cognitive function. Fifteen patient participants diagnosed with a brain tumour requiring radiotherapy will be recruited. Participants will undergo a remote, home-based 10-week personalised digital intervention using the CURATE.AI COR-Tx platform three times a week. Cognitive function will be assessed via a combined non-digital cognitive evaluation and a digital diagnostic session at five time points: preradiotherapy, preintervention and postintervention and 16-weeks and 32-weeks postintervention. Feasibility outcomes relating to acceptability, demand, implementation, practicality and limited efficacy testing as well as usability and user experience will be assessed at the end of the intervention through semistructured patient interviews and a study team focus group discussion at study completion. All outcomes will be analysed quantitatively and qualitatively.
Ethics and dissemination
This study has been approved by the National Healthcare Group (NHG) DSRB (DSRB2020/00249). We will report our findings at scientific conferences and/or in peer-reviewed journals.
Trial registration number
Keywords: radiotherapy, clinical trial, telemedicine, feasibility studies
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is a prospective, mixed-methods feasibility trial to inform a future clinical trial.
The behavioural component will provide insights into how to further develop the intervention for the patient population as well as how to scale for a larger multisite randomised control by including patients and study team members (clinicians/data team members).
This feasibility trial is a model for a decentralised trial in which patients can undergo treatment in the comforts of their own home and clinicians can monitor their progress.
The non-randomised single-arm feasibility trial does not simulate a randomised control trial as closely as a randomised pilot and is limited in informing on issues that may arise from the logistical process on a larger scale, including future decisions on determining eligibility criteria from a diverse patient population.
The digital nature of this intervention requires a higher level of technological literacy and skills which may be intimidating to some, introducing potential bias in recruitment and may have limited generalisability to other countries owing to cultural differences.
Introduction
Patients with brain tumours who undergo radiotherapy exhibit cognitive impairments throughout the course of their condition. These impairments often include decline in memory, attention and executive function, and they can be attributed to the tumour itself and/or side effects of its treatment.1–5 Cognitive deficits are reported to occur before radiotherapy treatment and in between 50% and 90% of adult patients 6 months after treatment.1 6–8 Such high prevalence, coupled with the increase in life expectancy of patients with brain tumour necessitates the need for appropriate strategies that preserve and improve cognitive functioning in brain tumour survivors.
To date, pharmacological interventions and cognitive rehabilitation therapy (CRT) have been the main approaches used to preserve and improve cognitive functioning in these patients.1 Pharmacological interventions typically include repurposed medications for cognitive functioning in other conditions, such as donepezil, armodafinil, modafinil and methylphenidate.1 However, evidence of the efficacy of these pharmacological interventions is limited and trial endpoints are often unmet.1 9–12 CRT involves neuropsychological interventions that are meant to augment various domains of cognitive function through the mechanism of neuroplasticity.1 CRTs can be provided directly to an individual or in group settings, conducted at home or in dedicated rehabilitation centres, and delivered face-to-face by a qualified clinician as pen-and-paper exercises or through computerised programmes.1 CRTs have shown promise with improvements in desired cognitive performance reported after their use in some patients.1 9 13 However, these findings are limited and inconsistently reported across studies, warranting further development of more robust CRTs for this population.
In both the pharmacological and CRT modalities, the reported treatment regimens are typically administered as a one-size-fits-all intervention with fixed doses or training intensities for the duration of the treatment for all patients. However, not only does the state of patient typically evolve over the duration of their condition, but each patient also experiences variable factors, such as tumour burden (eg, size, position and type), baseline cognitive abilities, treatment type and response (eg, efficacy, side effects, etc). As such, it remains possible that these uniform one-size-fits-all, fixed dose interventions are a large contributing factor to the suboptimal responses experienced by some patients.14 To be more effective, interventions that aim to preserve and improve cognitive functioning should treat each patient as an individual case, with the treatment tailored to that individual. Therefore, there is an urgent need to develop therapies that are personalised and that can dynamically adapt throughout the course of the condition for patients with brain tumour who undergo radiotherapy. Recently, artificial intelligence (AI) has established itself as a paradigm-shifting technology in healthcare with the potential to transform many aspects of patient care, if used appropriately.15 In particular, AI shows great potential in personalising care for patients from diagnosis to treatment selection and optimising intervention.14 16 As such, integrating AI into CRTs is a plausible solution to overcome the aforementioned challenges and pitfalls of the current one-size-fits-all, fixed-dose interventions.
Commonly, AI health technologies are developed from the big data paradigm in which population data and advanced statistical analyses are harnessed to diagnose and treat individual patients based on their demographics and disease history.17 These are often highly successful, but require large population datasets and substantial prior knowledge of the targeted condition in order to personalise care and avoid common biases.14 16 18 Further, while these methods can account for interpatient variability to identify appropriate care strategies, they have limited ability to account for the intrapatient variability of a dynamically changing patient state throughout the course of their condition.14 In contrast to the big data paradigm, small data paradigm AI health technologies framed to serve N-of-1 medicine require as little as only a patient’s own data to deliver personalised care by rapidly capturing their own response to a treatment over time.14 16 19 20 AI for N-of-1 medicine may be a favourable approach to dynamically modulate an intervention with the goal of optimising the efficacy for a patient over time19, and therefore has potential to improve interventions aimed at preserving and improving cognitive function in patients with brain tumour.
CURATE.AI is a small data, AI-derived, indication-agnostic and mechanism-independent platform that maps the relationship between an intervention intensity input and the phenotypic response output for a patient, using exclusively their own data.21 It is based on a previously established observation that a quadratic surface can closely represent the relationship between varying intervention intensities input and measurable phenotypic response output in a human system.22–27 Using this premise, the platform is prospectively calibrated by correlating patient-specific responses to a range of intervention intensities to create a patient’s individualised CURATE.AI profile. The prospectively calibrated profile is then paired with an intensity optimisation process to predict the patient’s phenotypic response output for a specified intensity input and to provide treatment intensity recommendations for optimised results. Importantly, the individualised CURATE.AI profile can be continuously recalibrated as the patient evolves throughout the course of their condition.
To date, the validity of CURATE.AI has been successfully demonstrated, both retrospectively and prospectively, for single drug optimisation of immunosuppression therapy28 and for combination drug optimisation of oncology therapy.29 30 Most recently, CURATE.AI was demonstrated as an integral part of a cognitive training platform to derive individualised learning profiles for young adults.31 More specifically, in the prospective, proof-of-concept study, the CURATE.AI platform was used to derive personalised learning profiles of healthy participants while they completed a multitasking cognitive training paradigm. The personalised learning profiles were generated by correlating a participant’s performance improvement to their performance at various intensities of the multitasking cognitive training paradigm. Overall, these profiles revealed substantial differences between individual performance at various intensity levels and demonstrated that individual-specific exposure to different training intensities is required to achieve maximum performance improvement during the multitasking cognitive training paradigm. The ability of the CURATE.AI platform to identify individualised training profiles provides the foundation for the optimisation of non-pharmaceutical therapies, such as CRTs.
Therefore, to address the urgent clinical need for a dynamic, personalised therapy that is effective in preserving and improving cognitive functioning in patients with brain tumour who undergo radiotherapy, we have developed the CURATE.AI COR-Tx platform as a digital therapeutic (DTx) with the potential to be used as a treatment and diagnostic tool. DTx are evidence-based software programmes that prevent, manage or treat a medical condition or disease that can be used independently or together with other modalities to deliver care directly to patients.32 DTx are typically easily deployable for at-home use and efficacy measurements (eg, scoring) can be given back to the individual as feedback, both of which may contribute to improved patient compliance and efficacy.16 The CURATE.AI COR-Tx platform combines CURATE.AI with tablet-ready digital cognitive training tasks as the interface. The CURATE.AI COR-Tx platform can dynamically optimise the treatment for the entire duration a patient’s care. This may result in improved cognitive function in these patients, as compared with traditional one-size-fits all, fixed-intensity CRTs, and potentially serve as an effective, interventional modality for patients with brain tumour who undergo radiotherapy. Further, as the CURATE.AI COR-Tx platform can be used throughout the duration of the condition, from initial diagnosis to after radiotherapy treatment, it is possible that performance measures captured by the digital cognitive training tasks may have the capacity to remotely establish cognitive function levels by detecting and monitoring a patient’s own ability and changes at dedicated time points and over time.
Objectives
The primary objective of this trial is to test the feasibility of the CURATE.AI COR-Tx platform as a digital intervention (DI) and a digital diagnostic (DD) for cognitive function in patients with brain tumour postradiotherapy. The secondary objective of this trial is to assess the usability of the CURATE.AI COR-Tx platform. Further exploratory objectives are to assess user experience (UX) with the CURATE.AI COR-Tx platform. Additionally, as the participants will use the CURATE.AI COR-Tx platform throughout the duration of their care, it is possible that the objective, quantifiable physiological and behavioural data collected from the DTx, known as digital biomarkers, may offer the ability to detect changes in cognitive function, such as improvement or decline, in these participants.33 Therefore, an additional exploratory outcome will include capturing and preliminary evaluation of potential digital biomarkers for cognitive function during the DI sessions. The results of this clinical feasibility trial will provide data required to design a definitive future multisite randomised control trial (RCT) to assess the efficacy of the CURATE.AI COR-Tx platform.
Methods and analysis
This trial is registered and published at ClinicalTrials.gov (NCT04848935). This protocol was prepared in adherence to the Consolidated Standards of Reporting Trials (CONSORT) extension for randomised pilot and feasibility trials reporting guidelines34 and Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT).35
Trial design
This is a prospective, single-centre, single-arm, mixed-methods feasibility clinical trial. The start date for this study was in April 2021 and is expected to run until April 2024. The outcome of this trial will provide data required to design a definitive, future, multisite RCT. Criteria for progression to a future larger trial will be based on the combined qualitative and quantitative feasibility of primary and secondary outcomes.
Study setting and participants
Fifteen patient participants will be recruited from the Department of Radiation Oncology, National University Cancer Institute Singapore (NCIS), part of the National University Health System (NUHS) in Singapore. Clinical investigators will recruit patients according to eligibility requirements during routine clinical visits prior to the planned commencement of partial or whole brain radiotherapy. Written informed consent will be gained from each participant prior to inclusion in this study. The rolling recruitment period for this study is between May 2021 and July 2023. Participants who are removed or who drop-out will not be replaced.
Eligibility criteria
Inclusion criteria: patient participants (1) with a neoplastic condition (benign or malignant) involving the brain or skull requiring radiotherapy (with or without chemotherapy); (2) aged ≥21 years; (3) Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 to 2; and (4) with a life expectancy of at least 6 months.
Exclusion criteria: patient participants (1) undergoing stereotactic radiosurgery (single fraction); (2) undergoing reirradiation to the same area of the brain; (3) unable to give informed consent; (4) who cannot understand spoken English language; (5) physically incapable of using a computer tablet (either due to vision loss or dominant hand weakness); and (6) who are pregnant or breastfeeding women.
Consent procedure
The lead clinical coordinator will meet potential participants at their outpatient appointment where they will be provided with a consent form, participant information leaflet and a verbal explanation of the study. Participants who are willing to take part in the study will sign a consent form and an appointment for baseline testing prior to commencement of their radiotherapy treatment will be scheduled (online supplemental material 1).
bmjopen-2023-077219supp001.pdf (140.6KB, pdf)
Intervention
CURATE.AI COR-Tx platform
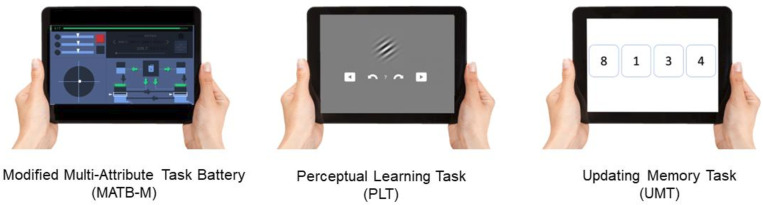
The CURATE.AI COR-Tx platform involves in-house developed tablet adaptations of multitasking, perceptual learning and executive processing digital cognitive training tasks which serve as the interface of the DI and DD. In the DI, the intensity of each task will be independently modulated by CURATE.AI, described in detail in subsequent sections, resulting in a dynamically personalised DTx CRT for each user. In the DD, the intensity of each task will be fixed and predefined for all users. One or more of the digital cognitive training tasks may serve as the interface for the CURATE.COR-Tx DI or DD. The digital cognitive training tasks of the CURATE.AI COR-Tx platform are depicted in figure 1 and described in detail below.
Figure 1.
CURATE.AI COR-Tx platform digital cognitive training tasks.
Modified multiattribute test battery
The multiattribute test battery (MATB) is a flight deck simulator originally developed by the National Aeronautics and Space Administration36 and further redefined by the United States Air Force.37 MATB is a multitasking paradigm that requires users to respond to the demands of four tasks simultaneously. The tasks require users to respond to auditory commands, track a target with a joystick, monitor system gauges for deviant readings and problem-solve to maintain fuel levels. The software was originally developed to be played on a computer with a monitor, joystick and headphones. In this current trial, participants will use a modified version of MATB (MATB-M) that our research team has developed. MATB-M is a tablet-ready, modernised and gamified adaptation of MATB that allows for remote operation (figure 1). MATB-M still replicates the functionality of MATB without the auditory command task and requires a user to complete multiple subtasks simultaneously. The intensity, or difficulty, of each subtask within MATB-M can be modulated, primarily by adjusting the frequency of critical events that demand evaluation and/or response. Performance is measured by a composite score of accuracies and reaction times in event solving of the individual tasks.
Perceptual learning task
The perceptual learning task is an online adaption of the orientation discrimination task with Gabor patches from Lengyel and Fiserfigure 1).38 Users are first shown a reference Gabor patch followed by a modified test Gabor patch that may be oriented clockwise or counter-clockwise. Users are required to indicate the direction of rotation. Difficulty can be adjusted by changing the degree of similarity between the two stimuli or by changing the stimuli’s visual contrast levels. Performance is measured by the accuracy of correct discriminations.
Updating memory task
The updating memory task is an online adaptation of the number memory task protocol from Morris and Jones (figure 1).39 In this task, a list of several numbers or letters will be presented serially for a designated time per item. Users are required to recall the last four items presented in the list. Difficulty can be adjusted by increasing the length of the list of items presented. Performance is measured by the accuracy of correctly recalled sequences.
CURATE.AI
CURATE.AI in this context refers to the CURATE.AI software used in the backend of the CURATE.AI COR-Tx platform that generates the calibrated, individualised profiles and subsequent training intensity recommendations for a DI training session. The Health Sciences Authority in Singapore classifies CURATE.AI as a Class B medical device (low to moderate risk), which is defined as all active therapeutic devices that are software, or which are intended to administer or exchange energy to, or with the human body. We have filed the accompanying Clinical Research Materials notification (CRM-N) under the National University of Singapore, for the intended purpose of providing training intensity recommendations within this clinical feasibility trial.
CURATE.AI recommendation
CURATE.AI will be used to provide training intensity recommendations for the DI component of the CURATE.AI COR-Tx platform. In CURATE.AI-guided training sessions, for each participant, CURATE.AI will undergo an initial calibration period with the aim of generating a personalised profile based on the treated participant’s own data only. During this initial calibration period, CURATE.AI will provide calibration-intent training recommendations to collect data on the participant’s phenotypic response, as measured by their performance, to a range of training intensities on a given DI task. CURATE.AI will then provide dynamic intensity recommendations for the remainder of the training session. CURATE.AI intensity recommendations will be within a prespecified intensity range of 13 difficulty levels. This process will be repeated for all CURATE.AI-guided training sessions in the DI and will continue until the end of the 10-week intervention.
Trial schedule and investigations
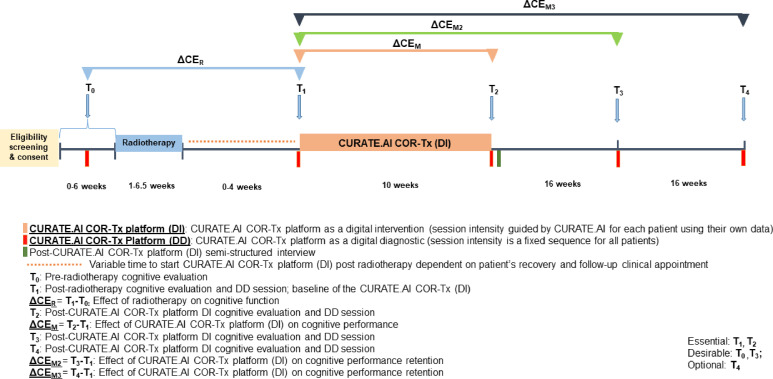
The feasibility SPIRIT trial schedule is summarised in figure 2 and investigations are described in detail below.
Figure 2.
Feasibility SPIRIT trial schedule and investigations. DD, digital diagnostic; DI, digital intervention; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
Participants will undergo a combined non-digital cognitive evaluation and a 10–15 min DD session at time points T0–T4.
T0: pre-radiotherapy combined non-digital cognitive evaluation and DD session.
T0 is a pre-radiotherapy session to evaluate cognitive function prior to radiotherapy. This may not always be possible due to the short time frame between the decision to undergo radiotherapy and its commencement. T0 is a desirable timepoint, but not essential.
T1: post-radiotherapy combined non-digital cognitive evaluation and DD session.
T1 is a post-radiotherapy and pre-DI session to evaluate baseline cognitive function prior to the DI. T1 is an essential timepoint.
CURATE.AI COR-Tx platform DI
Participants will complete three 12–15 min DI sessions per week (Monday, Wednesday and Friday) over 10 weeks for a total of 30 sessions. The CURATE.AI COR-Tx platform interface can be any of the three digital cognitive training tasks for a participant. Reminders about training sessions will be regularly sent to participants during the intervention from the clinical coordinator. These sessions will be completed at home on tablets provided by the study team.
T2: post-CURATE.AI COR-Tx platform DI combined non-digital cognitive evaluation and DD session.
T2 is a post-DI session to evaluate cognitive function after completion of the DI. Additionally, semistructured interviews exploring other feasibility outcomes (detailed in later sections) will occur within 5 days of DI completion. T2 is an essential timepoint.
T3 and T4: post-CURATE.AI COR-Tx platform DI combined non-digital cognitive evaluation and DD sessions.
T3 and T4 are post-DI sessions 16 and 32 weeks after the DI, respectively. These sessions evaluate mid-term and long-term retention of the effect of the DI on cognitive function. T3 is a desirable timepoint. T4 is an optional timepoint dependent on a participant’s patient status and condition.
Study completion
After completion of data collection and preliminary data analysis for all participants, a focus group meeting of all available trial team members will be held to discuss pertinent feasibility outcomes (detailed in subsequent sections of this protocol) and the potential expansion of a future multi-site RCT.
Sample size
We intend to recruit 15 participants for this study. As this is a feasibility clinical trial with no prior data, we did not perform formal sample size calculations. However, this sample size is based on the number of patients who can be practically and logistically recruited within the period of this feasibility trial that will allow for a reasonable signal to expand to a larger RCT.
Data collection, management and assessment
Outcomes
Primary outcomes
The primary outcome of this trial will be the feasibility of the CURATE.AI COR-Tx platform as both a DI and DD. Specific feasibility outcomes will be evaluated through qualitative and quantitative methods and analyses. Qualitative methods include 1 hour semistructured patient interviews and a trial team member focus group. The guide for the semistructured interviews is provided in online supplemental material 2. The specific aspects of feasibility, as defined by Bowen et al to be assessed in this trial include acceptability, demand, implementation, practicality and limited efficacy testing.40 Details of feasibility outcomes including definitions, measurement methods and analysis methods are provided in table 1.
Table 1.
Description of the feasibility outcomes to be assessed and how they will be collected and evaluated
| Aspect of feasibility40 | Feasibility outcome | Outcome definition | Methods for data collection | Methods for data analysis | Feasibility outcome evaluation according to CONSORT traffic light system34 | ||
| Green | Yellow | Red | |||||
| Acceptability | Patient acceptability | Patient perceived acceptability and suitability of the DI/DD | Data collected from semistructured interviews with patients | Thematic analysis | – | – | – |
| Trial team acceptability | Trial team perceived acceptability and suitability of the DI/DD | Data collected from focus group with trial team members at end of trial | Thematic analysis | – | – | – | |
| Randomisation appropriateness | Patient perceived appropriateness to hypothetically being randomised into a control group in a future clinical trial | Semistructured interviews | Thematic analysis | – | – | – | |
| Demand | Uptake | Percentage of successfully recruited patients from all patients approached and eligible for the study | Data collected during patient recruitment | Descriptive statistics | >50% | 10%–50% | <10% |
| Retention | Percentage of patients that complete the trial from all successfully recruited patients. | Data collected throughout trial completion. Reasons for drop-out will also be documented. | Descriptive statistics | >70% | 20%–70% | <20% | |
| Adherence (actual use) | Percentage of completed DI/DD sessions by patients at indicated timepoints | Data collected throughout trial completion | Descriptive statistics | >90% | 10%–90% | <10% | |
| Implementation | Success of DI execution | Percentage of DI sessions successfully performed at the indicated timepoints within this setting | Data collected throughout trial completion | Descriptive statistics | >70% | 10%–70% | <10% |
| Success of DD execution | Percentage of DD sessions successfully performed at the indicated timepoints within this setting | Data collected throughout trial completion | Descriptive statistics | >70% | 10%–70% | <10% | |
| CURATE.AI degree of execution | Percentage of patients to whom we successfully apply CURATE.AI profile analysis to | Data collected throughout trial completion | Descriptive statistics | >70% | 10%–70% | <10% | |
| Compliance response | The percentage of patients requiring and responding to reminders to complete DI/DD sessions | Data collected throughout trial completion | Descriptive statistics | – | – | – | |
| Practicality | DI/DD practicality | Trial team perception of the ability of patients to carry out DI/DD activities | Data collected from focus group with trial team members at end of trial | Thematic analysis | – | – | – |
| Logistical feasibility | Logistical considerations with current trial protocol that would need to be addressed or accounted for a future RCT | Data collected from focus group with trial team members at end of trial | Thematic analysis | – | – | – | |
| Limited-efficacy testing | DI limited efficacy | Exploratory analysis of the DI on the intended change in cognitive functioning pre–post intervention | Data collected from cognitive evaluation and DD sessions of the trial | Descriptive statistics | – | – | – |
| DD limited efficacy | Exploratory correlational analysis of outcomes between the digital cognitive training task and standard-of-care, gold standard cognitive evaluations | Data collected from cognitive evaluation and DD sessions of the trial | Descriptive statistics | – | – | – | |
CONSORT, Consolidated Standards of Reporting Trials; DD, digital diagnostic; DI, digital intervention; RCT, randomised control trial.
bmjopen-2023-077219supp002.pdf (71.8KB, pdf)
Secondary and exploratory outcomes
Secondary outcomes of this trial include the usability of CURATE.AI COR-Tx platform as a DI and DD in patients with brain tumour postradiotherapy. Usability will be evaluated qualitatively as part of the semi-structured interview session.
Exploratory outcomes will include the UX of the CURATE.AI COR-Tx platform as a DI and DD in patients with brain tumour postradiotherapy. UX will be evaluated qualitatively in the semistructured interview session. Further, as the participants will use the CURATE.AI COR-Tx platform throughout the duration of their care, it is possible that the objective, quantifiable physiological and behavioural data collected from the DTx, known as digital biomarkers, may offer the ability to detect changes in cognitive function and declines in these patients.33 Therefore, an additional exploratory outcome will include capturing and preliminary evaluation of potential digital biomarkers for cognitive function and decline during the DI sessions.
Patient-centred outcomes
Cognitive function
The non-digital cognitive evaluations will be used to assess different domains of cognitive functioning including memory, verbal fluency, executive function and global function and serve as the ‘gold standard’ comparison to evaluate the limited efficacy of the CURATE.AI CORTx platform as a DI and DD. All combined non-digital cognitive evaluations will be performed by a clinical neuropsychologist who will administer the test battery as recommended by the Radiotherapy Oncology Group.41 Memory impairment will be assessed using the Hopkins Verbal Learning Test.42 Verbal fluency will be assessed using the Controlled Oral Word Association Test.43 Executive function will be assessed using the Trail Making Test (Parts A and B).44 Global cognitive functioning will be assessed using the Mini-Mental State Examination.45 Patient-reported health-related quality of life will be assessed using the SF-36.46 Skill transfer will be assessed using the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog)47 48 and Cognitive Failures Questionnaire.49 Finally, the same clinical neuropsychologist will administer DD session which will be recorded via the CURATE.AI COR-Tx platform. Each combined non-digital cognitive evaluation and DD session will take approximately 1 hour to complete and will be performed at the Department of Radiation Oncology Clinic at NCIS.
Qualitative and statistical analysis
We will perform and report descriptive and inferential statistical analyses of the quantitative outcome measures. For qualitative outcomes thematic analysis will be used. All interviews and focus group sessions will be recorded and transcribed verbatim. Coding will be done manually. The analysis will follow the three stages: (1) data will be descriptively labelled (open coding); (2) labelled data will be grouped into categories based on literature (secondary coding); and (3) understanding the categories to create broader themes/assertions.50 We will not statistically analyse exploratory outcomes.
Data availability
Data generated and/or analysed during this clinical feasibility trial will be made available from the corresponding author on reasonable request.
Safety monitoring and data storage
Safety monitoring
The clinically trained principal investigator (PI) will oversee and monitor the conduct of this study to ensure the health and safety of participants and the validity and integrity of the data. Participants will be fully informed of the study requirements throughout the conduct of the study and should comply with the research protocol or be allowed to withdraw from participation. The PI will notify participants of any information relevant to their continued participation. Specifically, the PI will review the research protocol, evaluate the progress of the trial, including periodic assessments of data quality and timeliness, participant recruitment, accrual and retention, participant risk versus benefit, performance of the trial site and other factors that can affect study outcome. Scientific or therapeutic developments that may have an impact on the safety of the participants or the ethics of the study will be considered. The PI will make recommendations to the Domain-Specific Review Board (DSRB) and trial site concerning continuation or conclusion of the trial. The PI will protect the confidentiality of the trial data and the results of monitoring. CURATE.AI COR-Tx will only recommend the training intensity within the pre-specified intensity.
Safety reporting and monitoring
Adverse events (AEs) and serious adverse events (SAEs) will be monitored and recorded. All AEs will be recorded on the patient’s Case Report Form (CRF) from date of informed consent to 30 days following the last therapy session or initiation of new therapy, whichever occurs first. All treatment-related AEs will be followed until resolution of or until initiation of new therapy, whichever occurs first. During the long-term follow-up period, only secondary malignancies will be captured as AE. For both AEs and SAEs, the investigator will provide a record of the start and stop dates of the event, the action taken with study treatment as a result of the event (eg, discontinuation or reduction of study treatment) and outcome of the event. In the event of a possible study treatment-related AE, the investigator will to the best of his/her ability to assess its relationship to the study treatment. If an AE is considered serious, both the AE page/screen of the CRF and the SAE Report Form will be completed.
Data storage
Participants will interact with the CURATE.AI COR-Tx platform on trial provided tablets. Participant-identifying information (name, contact number, email) and the data linking subject identifiers and the subject identification codes will be collected and stored on one of the laboratory password-protected computers, which are kept in locked office rooms by the clinical team, separately from the research data to ensure that participants cannot be individually matched to their data. Clinical data will be stored on the Research Electronic Data Capture (REDCap) platform, a secure web application for building and managing online databases compliant with 21 Code of Federal Regulations (CFR) Part 11, Federal Information Security Management Act (FISMA), Health Insurance Portability and Accountability Act (HIPAA) and General Data Protection Regulation (GDPR), purposefully built to support online and offline data capture for research. While the study is ongoing, the deidentified (coded) research data will be retrieved from REDCap by the data analysis team and stored on one of the laboratory password-protected computers, which are kept in locked office rooms. Participants will be provided with a unique account and password to access sessions on the CURATE.AI COR-Tx platform. Only their own performance data will be stored within their unique account and on the secure cloud platform. Only the technical team will have access to the CURATE.AI COR-Tx platform performance data. Only the PI and collaborators will have access to the de-identified trial data.
Audio recordings and transcripts (with no identifiers revealed) of the semistructured interviews will be coded and stripped of identifying information at the earliest opportunity to ensure confidentiality of the participants. Participant-identifying information will be discarded on the completion of the research. Research data will be kept for future meta-analyses (including power analyses) and other occasions when the original data need to be referenced. These data will be retained for at least 10 years.
Patient and public involvement
This feasibility clinical trial was designed without patient and public involvement. However, this feasibility clinical trial includes a mixed-methods approach including semistructured patient participant interviews with aims to explore acceptability, usability and UX of the CURATE.AI COR-Tx platform as a DI and DD. The valuable input we will receive from these patient participants will be incorporated into the design of a future RCT.
Ethics and dissemination
This study has been approved by the National Healthcare Group (NHG) DSRB, reference: DSRB2020/00249. Clinical investigators will explain the protocol and obtain written, informed consent from patients as per the protocol prior to taking part in the study. We will report our findings at scientific conferences and/or in peer-reviewed journals. We will not publish any personal health identifiers.
Supplementary Material
Acknowledgments
We would like to thank Jason Labbe for his assistance in the development of CURATE.AI COR-Tx platform and C&B for enabling a collaborative environment.
Footnotes
Twitter: @AlexandriaRemus, @AgataBlasiak, @thedeanh
CLA, DH and BAV contributed equally.
Contributors: XT, AB, TK, DC, CLA, DH and BAV developed the study concept and initiated the project. AR, XT, GNSK, AB, TK, SV, LN, MR, DC, CLA, DH and BAV provided significant input into the development of the protocol. AR, SV, MR, QYC, FA, TKJ, YTT, AW, DC and BAV will implement the protocol and oversee the collection of the data. AR and XT drafted the manuscript, and all authors (AR, XT, GNSK, AB, TK, SV, LN, MR, QYC, FA, YR, TKJ, YTT, AW, DC, CLA, DH and BAV) read, contributed to and approved the final manuscript.
Funding: CLA, DH and BAV gratefully acknowledge funding from the Singapore Cancer Society [grant number SCS-GRA-2019-00063] for funding this current trial and had no influence on any part of this trial. DH gratefully acknowledges funding from National Research Foundation Singapore under its AI Singapore Programme [Award Number: AISG-GC-2019-002], and the Singapore Ministry of Health’s National Medical Research Council under its Open Fund- Large Collaborative Grant ('OF-LCG') [grant number MOH-OFLCG18May-0028], Institute for Digital Medicine (WisDM) Translational Research Programme [grant number R-719-000-037-733] at the Yong Loo Lin School of Medicine, National University of Singapore, Ministry of Education Tier 1 FRC Grant [grant number R-397-000-333-114] and the Next-Generation Brain-Computer-Brain Platform – A Holistic Solution for the Restoration & Enhancement of Brain Functions (NOURISH) project from the RIE2020 ADVANCED MANUFACTURING AND ENGINEERING (AME) PROGRAMMATIC FUND [grant number A20G8b0102/A-0002199-02-00]. All funders have no influence on the study design, collection, management, analysis interpretation of data, writing of the report and decision to submit the report for publication.
Competing interests: AB, TK, CLA and DH are coinventors of previously filed pending patents on artificial intelligence-based therapy development. DH and TK are shareholders of KYAN Therapeutics, which has licensed intellectual property pertaining to AI-based oncology drug development and personalised medicine.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Coomans MB, van der Linden SD, Gehring K, et al. Treatment of cognitive deficits in brain tumour patients: current status and future directions. Curr Opin Oncol 2019;31:540–7. 10.1097/CCO.0000000000000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol 2004;3:159–68. 10.1016/S1474-4422(04)00680-5 [DOI] [PubMed] [Google Scholar]
- 3.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. JCO 2004;22:157–65. 10.1200/JCO.2004.05.128 [DOI] [PubMed] [Google Scholar]
- 4.Lo SS, Teh BS, Jiang G-L, et al. Controversies in radiation oncology. In: Lo SS, Teh BS, Jiang G-L, eds. Brain Metastases BT - Controversies in Radiation Oncology. Cham: Springer International Publishing, 2020: 211–40. 10.1007/978-3-319-51196-2 [DOI] [Google Scholar]
- 5.Li J, Bentzen SM, Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys 2008;71:64–70. 10.1016/j.ijrobp.2007.09.059 [DOI] [PubMed] [Google Scholar]
- 6.Greene-Schloesser D, Robbins ME, Peiffer AM, et al. Radiation-induced brain injury: a review. Front Oncol 2012;2:73. 10.3389/fonc.2012.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer CK, McKee N, Case LD, et al. Mild cognitive impairment in long-term brain tumor survivors following brain irradiation. J Neurooncol 2019;141:235–44. 10.1007/s11060-018-03032-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tucha O, Smely C, Preier M, et al. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery 2000;47:324–33. 10.1097/00006123-200008000-00011 [DOI] [PubMed] [Google Scholar]
- 9.Cramer CK, Cummings TL, Andrews RN, et al. Treatment of radiation-induced cognitive decline in adult brain tumor patients. Curr Treat Options Oncol 2019;20:42. 10.1007/s11864-019-0641-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapp SR, Case LD, Peiffer A, et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. JCO 2015;33:1653–9. 10.1200/JCO.2014.58.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler JM, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-Threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys 2007;69:1496–501. 10.1016/j.ijrobp.2007.05.076 [DOI] [PubMed] [Google Scholar]
- 12.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429–37. 10.1093/neuonc/not114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol 2009;27:3712–22. 10.1200/JCO.2008.20.5765 [DOI] [PubMed] [Google Scholar]
- 14.Ho D, Quake SR, McCabe ERB, et al. Enabling technologies for personalized and precision medicine. Trends Biotechnol 2020;38:497–518. 10.1016/j.tibtech.2019.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J 2019;6:94–8. 10.7861/futurehosp.6-2-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho D, Teo G. Digital medicine – the new frontier for AI in healthcare. Advanced Therapeutics 2020;3:2000015. 10.1002/adtp.202000015 [DOI] [Google Scholar]
- 17.Mehta N, Pandit A, Shukla S. Transforming healthcare with big data analytics and artificial intelligence: a systematic mapping study. J Biomed Inform 2019;100:103311. 10.1016/j.jbi.2019.103311 [DOI] [PubMed] [Google Scholar]
- 18.Hekler EB, Klasnja P, Chevance G, et al. Why we need a small data paradigm. BMC Med 2019;17:133. 10.1186/s12916-019-1366-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egermark M, Blasiak A, Remus A, et al. Overcoming pilotitis in digital medicine at the intersection of data, clinical evidence, and adoption. Advanced Intelligent Systems 2022;4. 10.1002/aisy.202200056 [DOI] [Google Scholar]
- 20.Ho D. Artificial intelligence in cancer therapy. Science 2020;367:982–3. 10.1126/science.aaz3023 [DOI] [PubMed] [Google Scholar]
- 21.Blasiak A, Khong J, Kee T. CURATE.AI: optimizing personalized medicine with artificial intelligence. SLAS Technology 2020;25:95–105. 10.1177/2472630319890316 [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Lee D-K, Chen K-Y, et al. Mechanism-independent optimization of combinatorial nanodiamond and unmodified drug delivery using a Phenotypically driven platform technology. ACS Nano 2015;9:3332–44. 10.1021/acsnano.5b00638 [DOI] [PubMed] [Google Scholar]
- 23.Al-Shyoukh I, Yu F, Feng J, et al. Systematic quantitative characterization of cellular responses induced by multiple signals. BMC Syst Biol 2011;5:88. 10.1186/1752-0509-5-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohd Abdul Rashid MB, Toh TB, Silva A, et al. Identification and optimization of combinatorial glucose metabolism inhibitors in hepatocellular Carcinomas. SLAS Technology 2015;20:423–37. 10.1177/2211068215579612 [DOI] [PubMed] [Google Scholar]
- 25.Weiss A, Ding X, van Beijnum JR, et al. Rapid optimization of drug combinations for the optimal angiostatic treatment of cancer. Angiogenesis 2015;18:233–44. 10.1007/s10456-015-9462-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsutsui H, Valamehr B, Hindoyan A, et al. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat Commun 2011;2:167. 10.1038/ncomms1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong PK, Yu F, Shahangian A, et al. Closed-loop control of cellular functions using combinatory drugs guided by a stochastic search algorithm. Proc Natl Acad Sci USA 2008;105:5105–10. 10.1073/pnas.0800823105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarrinpar A, Lee D-K, Silva A, et al. Individualizing liver transplant immunosuppression using a phenotypic personalized medicine platform. Sci Transl Med 2016;8. 10.1126/scitranslmed.aac5954 [DOI] [PubMed] [Google Scholar]
- 29.Pantuck AJ, Lee D, Kee T, et al. Modulating BET bromodomain inhibitor ZEN-3694 and Enzalutamide combination dosing in a metastatic prostate cancer patient using CURATE.AI, an artificial intelligence platform. Advanced Therapeutics 2018;1:1800104. 10.1002/adtp.201800104 [DOI] [Google Scholar]
- 30.Lee D-K, Chang VY, Kee T, et al. Optimizing combination therapy for acute lymphoblastic leukemia using a phenotypic personalized medicine digital health platform: retrospective optimization Individualizes patient regimens to maximize efficacy and safety. SLAS Technology 2017;22:276–88. 10.1177/2211068216681979 [DOI] [PubMed] [Google Scholar]
- 31.Kee T, Weiyan C, Blasiak A, et al. Harnessing CURATE.AI as a digital therapeutics platform by identifying N-of-1 learning trajectory profiles. Advanced Therapeutics 2019;2:1900023. 10.1002/adtp.201900023 [DOI] [Google Scholar]
- 32.Digital Therapeutics Alliance . Digital therapeutics definition and core principles. 2019. Available: https://dtxalliance.org/wp-content/uploads/2019/11/DTA_DTx-Definition-and-Core-Principles.pdf
- 33.Babrak LM, Menetski J, Rebhan M, et al. Traditional and digital biomarkers: two worlds apart Digit Biomark 2019;3:92–102. 10.1159/000502000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comstock JR. The multi-attribute task battery for human operator workload and strategic behavior research Microform Va. Springfield, Va: National Aeronautics and space administration, Langley research center, National technical information service, distributor, 1992. [Google Scholar]
- 37.Miller WD, Schmidt KD, Estepp JR, et al. An updated version of the U.S Air Force Multi-Attribute Task Battery (AF-MATB). 2014.
- 38.Lengyel G, Fiser J. The relationship between initial threshold, learning, and generalization in perceptual learning. J Vis 2019;19:28. 10.1167/19.4.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris N, Jones DM. Memory updating in working memory: the role of the central executive. Br J Psychol 1990;81:111–21. 10.1111/j.2044-8295.1990.tb02349.x [DOI] [Google Scholar]
- 40.Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med 2009;36:452–7. 10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wefel JS, Pugh SL, Armstrong TS, et al. Neurocognitive function (NCF) outcomes in patients with glioblastoma (GBM) enrolled in RTOG 0825. JCO 2013;31(15_suppl):2004. 10.1200/jco.2013.31.15_suppl.2004 [DOI] [Google Scholar]
- 42.Brandt J. The Hopkins verbal learning test: development of a new memory test with six equivalent forms. Clinical Neuropsychologist 1991;5:125–42. 10.1080/13854049108403297 [DOI] [Google Scholar]
- 43.Kreutzer JS, DeLuca J, Caplan B. Encyclopedia of clinical Neuropsychology. In: Kreutzer JS, DeLuca J, Caplan B, eds. Controlled Oral Word Association Test BT - Encyclopedia of Clinical Neuropsychology. New York, NY: Springer, 2011: 703–6. 10.1007/978-0-387-79948-3 [DOI] [Google Scholar]
- 44.Salthouse TA. What cognitive abilities are involved in trail-making performance Intelligence 2011;39:222–32. 10.1016/j.intell.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Folstein MF, Folstein SE, McHugh PR. Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 46.Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Medicine 2016;4:205031211667172. 10.1177/2050312116671725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner LI, Lai JS, Cella D, et al. Chemotherapy-related cognitive deficits: development of the FACT-Cog instrument. Ann Behav Med 2004;27.(Suppl 10) [Google Scholar]
- 48.Wagner LI, Sweet J, Butt Z, et al. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy-cognitive function instrument. J Support Oncol 2009;7:W32–9. [Google Scholar]
- 49.Broadbent DE, Cooper PF, FitzGerald P, et al. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol 1982;21:1–16. 10.1111/j.2044-8260.1982.tb01421.x [DOI] [PubMed] [Google Scholar]
- 50.Lindlof TR, Taylor BC. Qualitative communication research methods. 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-077219supp001.pdf (140.6KB, pdf)
bmjopen-2023-077219supp002.pdf (71.8KB, pdf)
Data Availability Statement
Data generated and/or analysed during this clinical feasibility trial will be made available from the corresponding author on reasonable request.