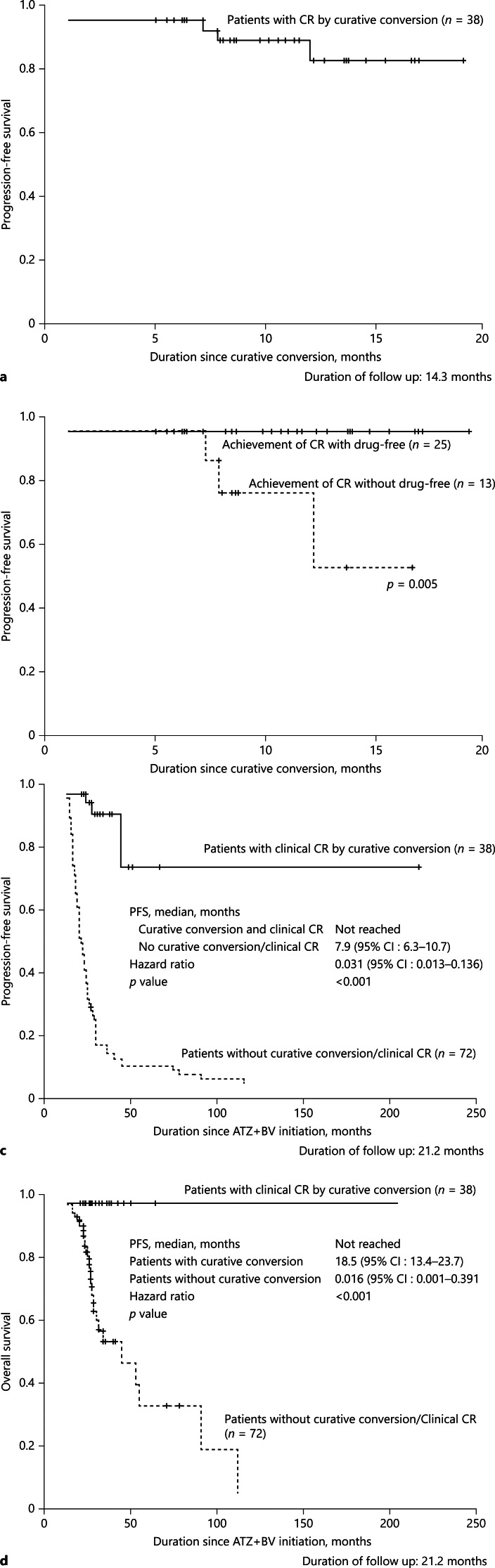
Fig. 4.
PFS (CR maintenance rate). a Median PFS of the 38 cases that achieved clinical CR was not reached. b There was no recurrence from the patients with CR and drug-free status. There were 3 recurrences, who received TACE alone (n = 2) or Atezo/Bev alone (n = 1). c Median PFS since atezolizumab plus bevacizumab initiation. Median PFS in patients who achieved clinical CR by curative conversion was much better than those who did not receive curative conversion or did not achieve CR (HR 0.031, p < 0.001). d Median OS since atezolizumab plus bevacizumab initiation. Median OS in patients who did not receive curative conversion or did not achieve clinical CR was 18.5 months (95% CI, 13.4–23.7). There was no death who achieved clinical CR by curative conversion. CR, complete response; ABC, atezolizumab plus bevacizumab followed by curative conversion; OS, overall survival; PFS, progression-free survival.