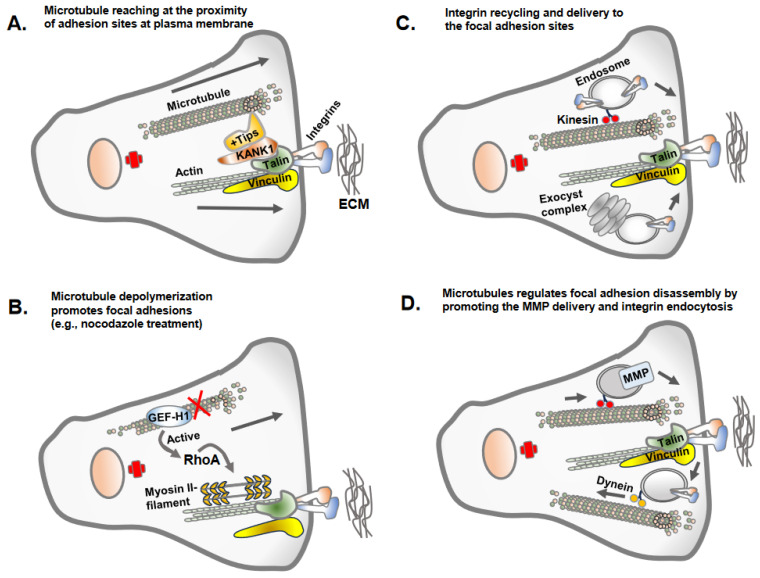
Figure 3.
Microtubule regulation of focal adhesions. There are different mechanisms by which the microtubule regulates focal adhesions. (A) Microtubules often do not reach the site of focal adhesions due to the dense actin cytoskeleton but make indirect contact via KANK1 or +Tips proteins, stabilizing the plus ends of the microtubules. (B) The key mechanism by which microtubule polymerization or growth inversely regulate focal adhesions is due to the activity of GEF-H1, the guanine exchange factor H1 for RhoA GTPases. GEF-H1 bound to microtubules is in an inactivated state. GEF-H1 is activated once released from depolymerized microtubules (e.g., nocodazole treatment of cells) and activates RhoA GTPase, which promotes actin polymerization and contractility and increased focal adhesion assembly. (C) Microtubules as a track for endosomal trafficking promote the delivery of the newly synthesized or recycling integrins to newly forming focal adhesion sites. An evolutionary conserved vesicle trafficking complex, exocyst, in association with microtubules may also deliver integrin molecules to focal adhesion sites. (D) Microtubules serve as a track for endosomal trafficking of matrix metalloprotease (e.g., MT1-MMP) at the site of focal adhesion, leading to the disassembly of the focal adhesions. The integrins once internalized from adhesion sites undergo endosomal trafficking along microtubules tracks.