Figure 2.
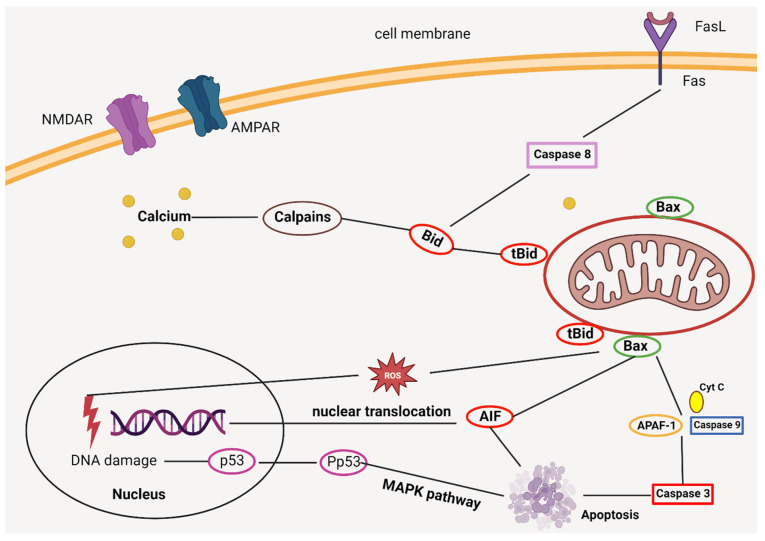
The intricate processes leading to ischemic neuronal cell death involve various molecular pathways and interactions. When NMDA receptors (NMDARs) and AMPA receptors (AMPARs) are stimulated, there is an elevation in the levels of calcium within the cell’s cytoplasm. This heightened calcium concentration triggers the activation of enzymes known as calpains and induces dysfunction within the mitochondria. Simultaneously, the binding of Fas ligands (FasL) to their counterparts, the Fas death receptors, sets off the activation of a protein called caspase 8. These activated calpains, together with caspase 8, collaborate to cleave a protein named Bid, transforming it into its truncated version, tBid. Once formed, tBid associates with another protein, Bax, on the mitochondrial membrane. This interaction is critical as it results in the creation of pores in the membrane, leading to the expulsion of several vital molecules: cytochrome c (Cyt c), apoptosis-inducing factor (AIF), and the harmful reactive oxygen species (ROS). After their release, both ROS and AIF relocate to the cell’s nucleus. Here, they play a pivotal role in damaging the DNA and initiating specific neuronal cell death pathways. A prime example of such a pathway is the phosphorylation of the protein p53, which, when phosphorylated (Pp53), activates the MAPK signaling route, pushing the cell towards apoptosis. Additionally, the expelled cytochrome c from the mitochondria plays a role outside its traditional function. In the cell’s cytoplasm, cytochrome c collaborates with the apoptotic protein activating factor-1 (APAF-1) and procaspase 9 to assemble into a complex known as the apoptosome. This structure is integral to the internal apoptotic pathway, further cementing the neuron’s fate towards programmed cell death.