Abstract
In this study, we explored the relationship between the platelet endothelial aggregation receptor 1 (PEAR1) polymorphisms, platelet reactivity, and clinical outcomes in patients with minor stroke or transient ischemic attack (TIA). Randomized controlled trial subgroups were assessed, wherein patients received dual antiplatelet therapy for at least 21 days. Platelet reactivity was measured at different time intervals. Genotypes were categorized as wild-type, mutant heterozygous, and mutant homozygous. Clinical outcomes were evaluated after 90 days. The rs12041331 polymorphism predominantly influenced adenosine diphosphate channel platelet activity, with the AA genotype displaying significantly lower residual platelet activity to the P2Y12 response unit (p < 0.01). This effect was more evident after 7 days of dual antiplatelet treatment (p = 0.016). Mutant A allele carriers had decreased rates of recurrent stroke and complex endpoint events but were more prone to bleeding (p = 0.015). The rs2768759 polymorphism majorly impacted arachidonic acid (AA) channel platelet activity, which was particularly noticeable in the C allele carriers. Our regression analysis demonstrated that rs12041331 AA + GA and rs2768759 CA predicted 90-day post-stroke bleeding. In conclusion, the PEAR1 polymorphisms rs12041331 and rs2768759 interfere with platelet aggregation and the performance of antiplatelet drugs. These genetic variations may contribute to bleeding events associated with minor stroke and TIA.
Keywords: PEAR1 gene, platelet aggregation, dual antiplatelet therapy, stroke, transient ischemic attack, genetic polymorphism
1. Introduction
The combination of aspirin with either clopidogrel or ticagrelor for 21 days is the standard therapy for minor stroke or Transient Ischemic Attack (TIA) [1,2,3]. A known challenge is high platelet reactivity (HPR), a phenomenon where platelet aggregation is not effectively suppressed by standardized medication, with an incidence rate of 5–65% [4,5]. The VerifyNow assay is commonly used to monitor platelet responses to antiplatelet drugs [6]. Currently, the causes of platelet hyperreactivity are uncertain, with factors such as age, sex, smoking habits, medication compliance, drug dosage, and genetic polymorphisms garnering significant attention. Numerous genes have been examined for their reactivity to aspirin, either alone or in concert with clopidogrel or ticagrelor [7,8,9,10]. Platelet Endothelial Aggregation Receptor 1 (PEAR1) is a platelet transmembrane protein that promotes platelet adhesion and aggregation, plays a role in thrombosis, and helps maintain platelet aggregation homeostasis [11]. Some studies have found a significant correlation between this gene locus and agonist-induced platelet aggregation activity [12]. This connection influences platelet reactivity to various agonists [13]. Since the COX1/thromboxane A2 pathway is strongly inhibited by aspirin, maximum aggregation relies on other secondary signaling pathways. Genetic variation in PEAR1 is thus considered a critical determinant of residual platelet function during aspirin treatment and might be a crucial determinant of residual platelet function during antiplatelet therapy [11,14], thereby affecting the clinical outcomes of stroke and TIA patients. This study aims to evaluate the effectiveness of aspirin in conjunction with clopidogrel or ticagrelor for treating minor strokes or TIA. We will investigate the association between PEAR1 gene polymorphisms, namely rs12041331, rs2768759, and rs56260937, platelet reactivity (as detected by VerifyNow), and the 90-day clinical outcome. The goal is to guide patients in taking effective measures to reduce platelet hyperreactivity, decrease the risk of bleeding, and improve clinical efficacy.
2. Materials and Methods
2.1. Study Population
A pre-designated subgroup analysis was conducted for the PRINCE trial, a randomized, multicenter, prospective, active-control, open-label trial with blinded endpoints. The rationale and design of the PRINCE trial have been previously outlined [15]. In brief, the trial randomly assigned patients with acute minor ischemic stroke (with an NHISS score ≤ 3) or moderate- to high-risk TIA (those with an ABCD2 score ≥4 or ≥50% neck or intracranial vascular stenosis explaining their clinical symptoms) to receive either a combination of ticagrelor and aspirin or clopidogrel and aspirin within 24 h of symptom onset. The patients were administered aspirin (300 mg on the first day and 100 mg daily from days 2–21) along with either ticagrelor (180 mg on the first day and 90 mg twice daily from days 2–90) or clopidogrel (300 mg on the first day and 75 mg daily from days 2–90). Patient enrollment commenced in August 2015, and the follow-up period concluded in June 2017. The PRINCE trial, which included 675 patients at 26 centers, is registered under the number NCT02506140 on ClinicalTrials.gov, accessed on 24 July 2020.
2.2. Platelet Reactivity Measurement
Patients were assessed at baseline and at 7 ± 2 days and 90 ± 7 days post-enrollment using the VerifyNow test. This test measured the Aspirin Reaction Unit (ARU) for aspirin and the P2Y12 Response Unit (PRU) for clopidogrel or ticagrelor. Platelet function tests were performed following the standardized procedure manual by qualified personnel at each study center. These personnel were blinded to the treatment assignments. While patients and investigators were aware of the study drug allocation, the data regarding platelet reactivity remained unavailable until the completion of the trial. To ensure the accuracy and reproducibility of these methods, we conducted two separate training sessions for all examiners at each study center.
2.3. PEAR1 Genotyping
Blood samples were delivered to Beijing Tiantan Hospital using cold chain logistics and subsequently stored at -80℃. We evaluated three single-nucleotide polymorphisms (SNPs) of the PEAR1 gene: rs12041331, rs2768759, and rs56260937. Genotyping was conducted using the Sequenom MassArray iPLEX platform (Sequenom, San Diego, CA, USA). In cases where the initial results were unclear, Sanger sequencing was performed using an ABI 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The call rate for each SNP exceeded 98.5%.
2.4. Outcome
The effectiveness of the treatment was assessed based on the incidence of stroke (ischemic or hemorrhagic) or composite vascular events (hemorrhagic or ischemic stroke, transient ischemic attack, myocardial infarction, or vascular death) within a 90-day period. Safety outcomes were identified according to the PLATO standard for bleeding events after 90 days [16].
2.5. Statistical Analysis
Continuous variables are presented as mean standard deviations, medians are displayed with interquartile ranges, and categorical variables are denoted as percentages. Student’s t-test or the Wilcoxon test were employed for the analysis of continuous variables, while the χ2 test was used for the categorical variables. Comparisons of baseline characteristics were made between the gene test group and the group excluded from the gene test, as well as comparisons regarding the incidence of major bleeding events. We evaluated the ARU and PRU values of different genotypes and compared the incidence of clinical endpoint events between the wild-type and mutant strains. Cox proportional hazards regression was used to assess differences in the incidence of clinical outcomes, and these were reported as 95% confidence interval (CI) hazard ratios (HR) over the 90-day follow-up period. To determine whether specific genotypic classes had distinct therapeutic effects, we examined the interaction effects of genotypic therapy. The χ2 test was utilized to verify the Hardy–Weinberg equilibrium. Statistical significance was established at p < 0.05. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Baseline Characteristics of Study Participants
Out of the 675 patients enrolled in the PRINCE trial, this subgroup analysis included 372 patients (55.1%). The baseline characteristics for both the included and excluded patient subgroups are presented in Table 1. Both groups showed a well-balanced distribution of baseline data, with no significant differences in age, sex, blood pressure, body mass index, antiplatelet drugs, concurrent diseases, smoking history, drug combination, randomization time, NHISS score, ABCD2 score, SSS-TOAST classification, creatinine clearance, blood glucose levels, or platelet count (p > 0.05). However, the groups differed significantly in terms of pulse rate and serum creatinine levels (p < 0.05). The subgroup participants had a median age of 61 years, with 311 (83.6%) patients experiencing minor stroke and 61 (16.4%) having TIA (Table 1).
Table 1 also delineates the baseline characteristics of patients who experienced a bleeding endpoint event (66 patients) and those who did not (302 patients). Both groups had an average age of 60.6 years and were primarily male. The mean systolic blood pressure at enrollment was between 151 and 155 mmHg. The distribution of medication history and previous disease history was well balanced between the two groups at the time of enrollment. Notably, the risk of bleeding in the ticagrelor/aspirin subgroup was higher than in the clopidogrel/aspirin group (p < 0.05), and the risk of bleeding in patients with minor stroke was higher than in those with TIA (p < 0.05).
Table 1.
Baseline characteristics and 90-day bleeding events of genetic subgroups in the PRINCE trial.
| Characteristic | Genetic Subgroups in the PRINCE Trial | p Value | 90-Day Bleeding Events in Genetic Subgroups | p Value | ||
|---|---|---|---|---|---|---|
| Included in the Genetic Subgroup (n = 372) | Excluded from the Genetic Subgroup (n = 303) | No-90-Day Bleeding Events (n = 302) | 90-Day Bleeding Events (n = 66) | |||
| Age (years) | ||||||
| Mean (standard deviation) | 60.6 (9.0) | 61.0 (8.4) | 0.481 | 60.6 (9.1) | 60.6 (8.5) | 0.983 |
| Median (interquartile range) | 61.0 (55.0–67.0) | 62 (54.0–67.0) | 61 (55–67) | 59 (53–67) | ||
| Female sex | 93(25.0) | 88 (29.0) | 0.238 | 73 (23.9) | 20 (30.3) | 0.273 |
| Systolic blood pressure (mmHg) | ||||||
| Mean (standard deviation) | 154.2 (22.3) | 152.9 (21.3) | 0.427 | 154.8 (22.5) | 151.5 (21.6) | 0.271 |
| Median (interquartile range) | 152 (140–170) | 151 (140–168) | 154.5 (140–170) | 150.5 (140–160) | ||
| Diastolic blood pressure (mm Hg) | ||||||
| Mean (standard deviation) | 88.8 (13.2) | 88.2 (12.6) | 0.519 | 89.0 (13.2) | 88.2 (13.4) | 0.669 |
| Median (interquartile range) | 88.5 (80–97) | 87.0 (80–95) | 89 (80–98) | 87 (80–96) | ||
| Body mass index * | ||||||
| Mean (standard deviation) | 25.1 (3.9) | 24.8 (3.7) | 0.306 | 25.3 (3.9) | 24.5 (3.6) | 0.157 |
| Median (interquartile range) | 25.0 (22.8–27.3) | 24.2 (22.5–27.3) | 25.0 (22.9–27.2) | 24.2 (21.5–27.5) | ||
| Pulse rate (beat/min; mean (SD)) | 76.8 (10.8) | 74.3 (10.8) | 0.003 | 77.0 (10.7) | 75.7 (11.2) | 0.379 |
| Antiplatelet therapy, n (%) | ||||||
| Clopidogrel + aspirin | 185 (49.7) | 151 (49.8) | 0.979 | 165 (53.9) | 22 (33.3) | 0.002 |
| Ticagrelor + aspirin | 187 (50.3) | 152 (50.2) | 141 (46.1) | 44 (66.7) | ||
| Medical history, n (%) | ||||||
| Hypertension | 233 (62.6) | 178 (58.8) | 0.303 | 189 (61.8) | 44 (66.7) | 0.455 |
| Dyslipidemia | 25(6.7) | 16(5.3) | 0.436 | 21(6.9) | 4(6.0) | 0.813 |
| Diabetes mellitus/Hyperglycemia (baseline > 11.1 mmol/L) | 92 (24.7) | 72 (23.7) | 0.790 | 78 (25.5) | 14 (21.2) | 0.465 |
| Ischemic stroke | 68 (18.3) | 53 (17.5) | 0.791 | 58 (19.0) | 10 (15.2) | 0.469 |
| Transient ischemic attack | 12 (3.2) | 6 (2.00) | 0.318 | 10 (3.3) | 2 (3.0) | 0.921 |
| Coronary artery disease | 25 (6.7) | 26 (8.6) | 0.363 | 19 (6.2) | 6 (9.1) | 0.396 |
| Ex-smoker | 32 (8.6) | 19 (6.3) | 28 (9.2) | 4 (6.1) | ||
| Drug use before randomization (No (%)) | ||||||
| Proton pump inhibitor | 2 (0.5) | 3 (1.0) | 0.495 | 1 (0.3) | 1 (1.5) | 0.231 |
| Statin | 33 (8.9) | 33 (10.9) | 0.380 | 27 (8.8) | 6 (9.1) | 0.945 |
| Aspirin | 88 (23.7) | 58 (19.1) | 0.157 | 69 (22.6) | 19 (28.8) | 0.279 |
| Clopidogrel | 7 (1.9) | 8 (2.6) | 0.506 | 6 (2.0) | 1 (1.5) | 0.809 |
| Ticagrelor | 0 (0.0) | 0 (0.0) | -- | 0 (0.0) | 0 (0.0) | -- |
| Anticoagulants | 0 (0.0) | 0 (0.0) | -- | 0 (0.0) | 0 (0.0) | -- |
| Defibrase | 1 (0.3) | 0 (0.0) | 0.366 | 1 (0.33) | 0 (0.0) | 0.642 |
| Time to randomization after onset of symptoms (h; median (IQR)) | 13.9(6.6) | 14.4(6.8) | 0.366 | 13.8(6.6) | 14.9 (6.7) | 0.220 |
| Time to randomization after onset of symptoms (No (%)) | ||||||
| <12 h | 214 (57.5) | 178 (58.8) | 0.750 | 132 (43.1) | 26 (39.4) | 0.577 |
| ≥12 h | 158 (42.5) | 125 (41.3) | 174 (56.9) | 40 (60.6) | ||
| Qualifying event (No (%)) | ||||||
| Minor stroke | 311 (83.6) | 253 (83.5) | 0.971 | 250 (81.7) | 61 (92.4) | 0.033 |
| Transient ischemic attack | 61 (16.4) | 50 (16.5) | 56 (18.3) | 5 (7.6) | ||
| Baseline NIHSS score (median (IQR)) | 1.6 (1.1) | 1.9 (1.0) | 0.074 | |||
| Baseline ABCD2 (median (IQR)) | 4.85 (0.9) | 4.62 (0.8) | 0.177 | 4.9 (1.0) | 4.8 (0.4) | 0.898 |
| SSS-TOAST stroke subtype (No (%)) | ||||||
| Large artery atherosclerosis | 169 (54.3) | 135 (53.4) | 0.999 | 133 (53.2) | 36 (59.0) | 0.908 |
| Non-Large artery atherosclerosis | 142 (45.7) | 118 (46.6) | 117 (46.8) | 25 (41.0) | ||
| Serum creatinine, μmol/L | 72.7 (18.8) | 68.9 (18.7) | 0.009 | 73.4 (18.7) | 69.4 (18.9) | 0.114 |
| Plasma glucose, mmol/L | 6.2 (2.2) | 6.2 (2.4) | 0.854 | 6.3 (2.2) | 6.5 (2.4) | 0.988 |
| platelet count | 220.4 (57.6) | 217.3 (61.4) | 0.501 | 220.2 (57.9) | 221 (56.9) | 0.914 |
* Data are represented as either the median with its interquartile range or as a count (percentage). NIHSS refers to the National Institute of Health Stroke Scale; SSS-TOAST stroke subtype refers to the Stroke Subtype as per the Stop Stroke Study Trial of Org 10172 in Acute Stroke Treatment Stroke Etiology Classification).
3.2. PEAR1 Gene Test Results
Individual variants were eliminated if the genotyping rate was less than 95%. The distribution of polymorphisms for rs12041331, rs2768759, and rs56260937 complied with the Hardy–Weinberg equilibrium (p > 0.05), suggesting that this patient population exhibited a certain degree of population representativeness (Table 2).
Table 2.
Results of PEAR1 gene analysis.
| SNP Reference Number | Allele | Genotyping | Frequency of Allele Distribution | HWE p-Value | ||
|---|---|---|---|---|---|---|
| Wild-Type Homozygote (No (%)) | Heterozygous Mutation (No (%)) | Homozygous Mutation (No (%)) | ||||
| rs12041331 | G/A | 127 | 190 | 55 | G 59.7% A 40.3% | 0.732 |
| rs2768759 | A/C | 324 | 44 | 0 | A 94.02% C 5.78% | 0.419 |
| rs56260937 | C/T | 176 | 155 | 39 | C 68.5% T 31.5% | 0.666 |
HWE: Hardy–Weinberg equilibrium.
3.3. Relationship between Platelet Aggregation Pre- and Post-Treatment and Various SNPs
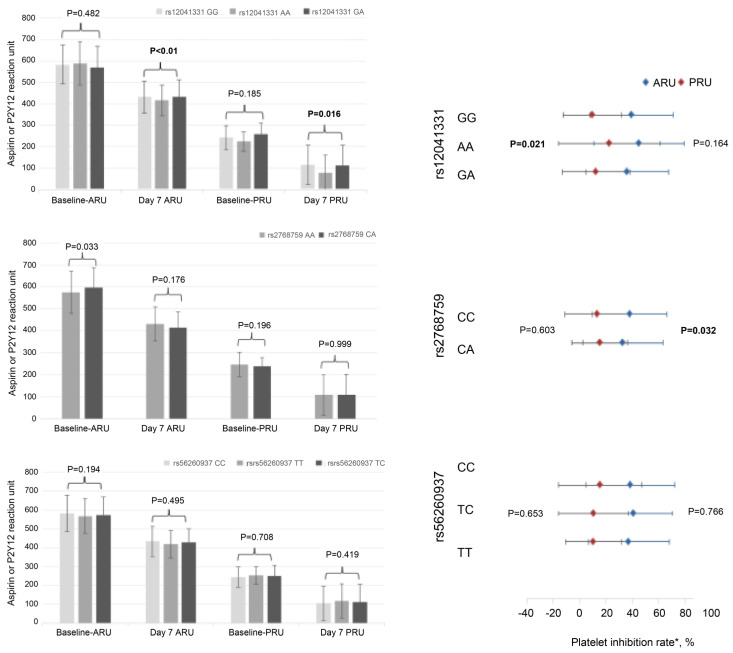
Carriers of the rs12041331 AA genotype displayed significantly lower platelet residual activity compared to the GG and GA genotypes at baseline as measured by PRU (p < 0.01). This difference became even more pronounced after seven days of dual antiplatelet drug treatment (p = 0.021). Notably, carriers of the A allele demonstrated higher platelet inhibition rates and significant differences in residual activity. These A allele carriers also exhibited greater platelet reactivity to ADP. A similar trend was observed for ARU, although the differences were not statistically significant. For rs2768759, the AA genotype was associated with significantly lower levels of platelet residual activity compared to the CA genotype (p = 0.033). Additionally, the platelet inhibition rate of ARU in the AA group was significantly higher than that in the CA group (p = 0.032). This suggests that the platelet reactivity of the C allele carriers to ARU was significantly greater. No substantial correlation was observed between the rs56260937 polymorphism and ARU or PRU levels. However, the rs12041331 and rs2768759 polymorphisms had notable impacts on platelet function and platelet aggregation and influenced the activity of antiplatelet drugs (refer to Figure 1).
Figure 1.
Analysis of platelet reactivity. The three genotypes of rs12041331 had different levels of PRU at baseline, day 7, and the platelet inhibition rates. For rs2768759, the platelet inhibition rate of ARU in the AA group was significantly higher than that in the CA group. ARU: Aspirin Reaction units; PRU: P2Y12 response unit. * Platelet Inhibition Rate: (baseline ARU/RRU−day 7 ARU/RRU) ÷ baseline ARU/RRU × 100.
3.4. Relationship between Various SNPs and Clinical Outcomes
Patients were categorized into groups based on the mutations in two single-nucleotide polymorphisms (SNPs): those carrying the wild-type allele (wild-type homozygote) and those carrying mutant alleles (mutant heterozygote and mutant homozygote). We then compared the occurrence of endpoint events between these groups. For both SNPs, there was no statistically significant difference in the incidence of endpoint events between patients with or without mutations (Table 3). However, those carrying the rs12041331 mutant allele demonstrated lower incidences of recurrent stroke, ischemic stroke, and multiple key events. Further, those carrying the ‘a’ allele at rs12041331 were more likely to experience bleeding events (p = 0.0146). Similarly, those carrying the ‘a’ allele at rs2768759 exhibited lower rates of recurrent stroke, ischemic stroke, and multiple key events. Additionally, carriers of the ‘a’ allele at rs12041331 showed a higher likelihood of bleeding events (p = 0.0112).
Table 3.
Analysis of clinical outcomes.
| Outcomes | rs12041331 (n = 372) | rs2768759 (n = 368) | rs56260937 (n = 360) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GG (n = 127) |
AA + AG (n = 245) | p Value | CA (n = 44) |
AA (n = 324) | p Value | CC (n = 176) |
CT + TT (n = 194) |
p Value | |
| Stroke | 12 (9.45) | 18 (7.35) | 0.480 | 4 (9.09) | 26 (8.02) | 0.808 | 18 (10.23) | 12 (6.19) | 0.155 |
| Composite events | 12 (9.45) | 19 (7.76) | 0.575 | 4 (9.09) | 27 (8.33) | 0.865 | 19 (10.80) | 12 (6.19) | 0.110 |
| Ischemic stroke | 12 (9.45) | 15 (6.12) | 0.241 | 4 (9.09) | 23 (7.10) | 0.634 | 15 (8.52) | 12 (6.19) | 0.388 |
| Major bleeding | 0 (0.00) | 3 (1.22) | 0.554 | 0 (0.00) | 3 (0.93) | 1.000 | 3 (1.70) | 0 (0.00) | 0.107 |
| Major or minor bleeding | 0 (0.00) | 3 (1.22) | 0.554 | 0 (0.00) | 3 (0.93) | 1.000 | 3 (1.70) | 0 (0.00) | 0.107 |
| Any bleeding | 14 (11.02) | 52 (21.22) | 0.015 | 2 (4.55) | 63 (19.44) | 0.011 | 32 (18.18) | 33 (17.01) | 0.767 |
3.5. Logistic Regression Analysis
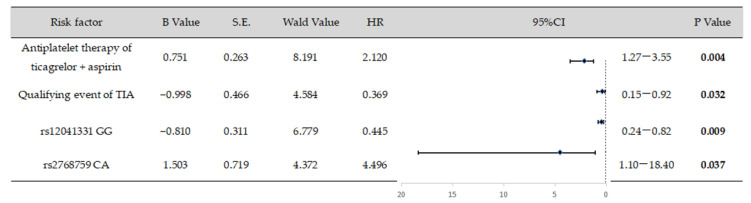
A logistic regression analysis was conducted to identify the risk factors for bleeding events. The findings indicated a positive correlation between bleeding events and the following independent risk factors: the antiplatelet treatment strategy employed by the ticagrelor and aspirin groups, the onset of minor stroke as an entry event, the AA + AG genotype at the rs12041331 locus, and the CA genotype at the rs2768759 locus (Figure 2). These factors were each independently associated with an increased risk of bleeding events.
Figure 2.
Logistic regression analysis of risk factors for bleeding events. Risk factors such as the ticagrelor and aspirin antiplatelet strategy, the initial event of mild stroke, and the presence of the AA + AG genotype at rs12041331 and the CA genotype at rs2768759 show a positive correlation with bleeding events. These factors are considered independent risk predictors for bleeding events.
4. Discussion
A subgroup analysis of the PRINCE trial confirmed that polymorphisms of the PEAR1 gene, specifically SNPs rs12041331 and rs2768759, influence platelet function, aggregation, and the effectiveness of antiplatelet drugs. There was a statistically significant difference in bleeding event incidence between patients with and without these mutations, with carriers of the rs12041331 and rs2768759 alleles experiencing an elevated risk of bleeding.
PEAR1 gene variants have been linked to platelet aggregation induced by various agonists [17,18] and the responses to several antiplatelet drugs, including aspirin [10,17,19], clopidogrel [20], and ticagrelor [21]. Since the COX1/thromboxane A2 pathway is strongly inhibited by aspirin, maximum aggregation relies on other secondary signaling pathways. Genetic variation in PEAR1 is thus considered a critical determinant of residual platelet function during aspirin treatment [11]. Notably, the intron variant rs12041331 in PEAR1 is a significant genetic modifier of platelet reactivity. Polymorphisms in rs12041331 can cause variations in gene and protein expression in patients treated with aspirin [22,23,24]. The PEAR1 rs12041331 A allele has been significantly linked with reduced agonist-induced platelet aggregation in both African Americans and Europeans, accounting for approximately 15% of total phenotypic variation in platelet reactivity [18,25]. Our study found that platelets with different genotypes exhibited varying levels of baseline adenosine diphosphate (ADP) activity, and significant variations in ADP activity persisted even after dual antiplatelet intervention. The VerifyNow test corroborated that the PEAR1 rs12041331 polymorphism primarily affects the ADP channel. This finding aligns with a previous turbidimetric test, indicating that rs12041331 in PEAR1 impacts ADP-induced aggregation, much like arachidonic acid (AA). However, there was no significant correlation between AA and AA [26]. Unlike the GG and GA genotypes, the AA homozygotes of the intron rs12041331 of PEAR1 influence ADP-induced aggregation [27,28,29]. The rs12041331 polymorphism, resulting in a G to A substitution, has been associated with reduced PEAR1 expression [28]. Faraday et al. found that the major G allele is independently linked with higher platelet aggregation irrespective of aspirin therapy and that it does not alter the relationship between PRU and rs12041331 [30]. Moreover, the AA genotype of rs12041331 has been associated with an increased response to ticagrelor in healthy individuals [21]. Research on SNP rs2768759 is relatively sparse. Some studies have shown that the C allele of rs2768759, which responds to different agonists in healthy individuals, is linked to increased AA- and ADP-induced platelet aggregation. This association is stronger after antiplatelet drug administration, suggesting that the C allele is linked to natural platelet aggregation and reduced responsiveness to antiplatelet drugs [10]. The AA variant of rs2768759 was associated with lower platelet aggregation than the CA variant, but no significant difference was observed in AA- and ADP-induced platelet aggregation between these two genotypes [10,31]. Our study revealed that the gene polymorphism rs2768759 significantly differs from the baseline ARU and ARU differences, primarily affecting the AA channel, unlike rs12041331. C allele carriers had low ARU levels and high platelet inhibition rates after seven days of treatment. No significant differences were observed among the rs56260937 genotypes. However, the greater the difference in the TT genotype, the stronger the antiplatelet effect and the higher the bleeding risk. Consistent with previous studies [32], Kim et al. analyzed the effect of PEAR1 variants on platelet function by sequencing the 1Q21.1 region of the chromosomes in 104 subjects. Platelet function was assessed using a PAP-4 Aggregometer following stimulation with collagen, ADP, or epinephrine. The results showed that rs56260937 was significantly linked with increased platelet aggregation across all three agonist phenotypes.
Research regarding the association between rs12041331, a potent genetic determinant of PEAR1, and cardiovascular outcomes under antiplatelet therapy is sparse, and the literature contains inconsistent results [33,34,35,36]. In Lewis’s study, carriers of the rs12041331 A allele exhibited a significantly elevated risk of myocardial infarction compared to GG homozygotes among Caucasian subjects undergoing percutaneous coronary intervention. This factor was considered an independent risk factor for cardiovascular events and platelet response in patients receiving either aspirin alone or in combination with clopidogrel [35]. Conversely, a post hoc analysis of 13,547 participants in the ASPREE trial indicated no association between the genetic variant rs12041331 and cardiovascular events in a healthy elderly population of European descent taking low-dose aspirin, with no clinically significant bleeding being observed [36]. Additionally, a meta-analysis encompassing six studies with 9914 patients revealed a link between the A allele of rs12041331, the AA homozygote of rs2768759, and an increased risk of ischemic events in patients with coronary heart disease receiving aspirin and P2Y12 receptor inhibitors [34]. Moreover, carrying the small T allele of rs56260937 independently predicted revascularization events in patients undergoing percutaneous coronary intervention for acute myocardial infarction [37]. A study on bleeding events indicated that patients with the TT + CT genotype of PEAR1 rs41273215 exhibited a three-fold higher incidence of significant bleeding and higher platelet inhibition rates in ACS and AF patients [38].
Research investigating the relationship between PEAR1 and stroke is scant, and the results remain elusive. A single-center study in Shanghai suggested that the impact of the rs12041331 polymorphism on aspirin efficacy depended on the TOAST subtype. Patients with rs12041331 AA stroke with small-artery occlusion demonstrated the highest sensitivity to sole aspirin therapy, leading to favorable short-term functional outcomes [39]. Although this might appear inconsistent with cardiovascular research, it aligns with our study and the underlying biology. In our study, patients with the rs12041331 AA genotype experienced the most notable reduction in ARU or PRU following dual antiplatelet therapy, indicating the highest platelet activity; the rates of stroke recurrence, ischemic stroke, and complex endpoint events were lower, and bleeding events were more probable. Likewise, compared to the AA type, the CA type of rs2768759 showed a significant ARU decrease following antiplatelet therapy, strong drug responsiveness, and a notable statistical difference in bleeding events.
Our regression analysis revealed that the AA genotypes of rs12041331 and CA of rs2768759 were predictors of short-term bleeding events post-stroke. This study is the first to provide robust evidence regarding the outcomes of multiple SNPs and dual-antibody stroke therapy, especially with respect to their correlation with bleeding. However, the incidence of recurrent stroke and composite endpoint events was not statistically significant, warranting further research. Notably, some patients without CYP2C19 mutations still experience ischemic events. Aside from pharmacokinetic pathways, mutations in genes associated with platelet aggregation might also influence the effects of P2Y12 receptor inhibitors and aspirin.
The limited sample size of this study restricted a subgroup analysis of the potential impacts of different medication regimes. In addition to PEAR1 gene mutations, some studies have shown significant associations between CYP2C19, P2RY12, and ABCB1 genotypes and ischemic clinical outcomes. However, due to the paucity of research, this study did not consider these factors. Future studies analyzing polygenetic scores in different ethnic populations may enhance our understanding of the relationship between PEAR1 SNPs and clinical outcomes in stroke patients. Going forward, PEAR1 should be further investigated as a critical factor for managing treatment in stroke patients. Genetic testing and assessments of the PEAR1 polymorphism could guide platelet activity diagnosis and bleeding prediction and ultimately improve patient treatment processes.
5. Conclusions
The polymorphisms at the rs12041331 and rs2768759 sites of the PEAR1 gene influence platelet aggregation and can interfere with the efficacy of antiplatelet drugs. These polymorphisms could be crucial determinants of bleeding events associated with dual antiplatelet therapy for minor stroke and TIA.
Acknowledgments
We extend our sincere gratitude to all hospitals that participated in the study, and we thank all the participants and their families for their cooperation and participation.
Author Contributions
Y.X. and D.Y. conceptualized and designed the study and drafted the initial manuscript. D.Z., L.J., W.C., X.Z., L.L. and Y.W. (Yongjun Wang) gathered the data and contributed to the study design. H.Y., Y.P. and Y.W. (Yicong Wang) were involved in the statistical analyses. Y.W. (Yilong Wang) and Y.P. contributed to the study design and assisted in drafting the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Beijing Tiantan Hospital (ethical approval number KY2014-048-03).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design, execution, interpretation, or writing of the study.
Funding Statement
This research article was funded by the National Natural Science Foundation of China (Grant No. 81825007), Beijing Outstanding Young Scientist Program (Grant No. BJJWZYJH01201910025030), Capital’s Funds for Health Improvement and Research (Grant No. 2022-2-2045, National Key R&D Program of China (Grant Nos. 2022YFF1501500, 2022YFF1501501, 2022YFF1501502, 2022YFF1501503, 2022YFF1501504 and 2022YFF1501505), Youth Beijing Scholar Program (Grant No. 010), Beijing Laboratory of Oral Health (Grant No. PXM2021_014226_000041), Beijing Talent Project—Class A: Innovation and Development (Grant No. 2018A12), National Ten—Thousand Talent Plan—Leadership of Scientific and Technological Innovation, and National Key R&D Program of China (Grant Nos. 2017YFC1307900 and 2017YFC1307905).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wang Y., Wang Y., Zhao X., Liu L., Wang D., Wang C., Wang C., Li H., Meng X., Cui L., et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N. Engl. J. Med. 2013;369:11–19. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 2.Farrant M., Easton J.D., Adelman E.E., Cucchiara B.L., Barsan W.G., Tillman H.J., Elm J.J., Kim A.S., Lindblad A.S., Palesch Y.Y., et al. Assessment of the end point adjudication process on the results of the platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trial: A secondary analysis. JAMA Netw. Open. 2019;2:e1910769. doi: 10.1001/jamanetworkopen.2019.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Meng X., Wang A., Xie X., Pan Y., Johnston S.C., Li H., Bath P.M., Dong Q., Xu A., et al. Ticagrelor versus Clopidogrel in CYP2C19 Loss-of-Function Carriers with Stroke or TIA. N. Engl. J. Med. 2021;385:2520–2530. doi: 10.1056/NEJMoa2111749. [DOI] [PubMed] [Google Scholar]
- 4.Maree A.O., Fitzgerald D.J. Variable Platelet Response to Aspirin and Clopidogrel in Atherothrombotic Disease. Circulation. 2007;115:2196–2207. doi: 10.1161/CIRCULATIONAHA.106.675991. [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo D.J. Variability in responsiveness to oral antiplatelet therapy. Am. J. Cardiol. 2009;103:27A–34A. doi: 10.1016/j.amjcard.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Venketasubramanian N., Agustin S.J., Padilla J.L., Yumul M.P., Sum C., Lee S.H., Ponnudurai K., Gan R.N. Comparison of Different Laboratory Tests to Identify ‘Aspirin Resistance’ and Risk of Vascular Events Among Ischaemic Stroke Patients: A Double-Blind Study. J. Cardiovasc. Dev. Dis. 2022;9:156. doi: 10.3390/jcdd9050156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuern C.S., Schwab M., Gawaz M., Geisler T. Platelet Pharmacogenomics. J. Thromb. Haemost. 2010;8:1147–1158. doi: 10.1111/j.1538-7836.2010.03791.x. [DOI] [PubMed] [Google Scholar]
- 8.Feher G., Feher A., Pusch G., Lupkovics G., Szapary L., Papp E. The Genetics of Antiplatelet Drug Resistance. Clin. Genet. 2009;75:1–18. doi: 10.1111/j.1399-0004.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 9.Agúndez J.A., Martínez C., Pérez-Sala D., Carballo M., Torres M.J., García-Martín E. Pharmacogenomics in Aspirin Intolerance. Curr. Drug Metab. 2009;10:998–1008. doi: 10.2174/138920009790711814. [DOI] [PubMed] [Google Scholar]
- 10.Herrera-Galeano J.E., Becker D.M., Wilson A.F., Yanek L.R., Bray P., Vaidya D., Faraday N., Becker L.C. A novel variant in the platelet endothelial aggregation receptor-1 gene is associated with increased platelet aggregability. Arterioscler. Thromb. Vasc. Biol. 2008;28:1484–1490. doi: 10.1161/ATVBAHA.108.168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanda N., Bao M., Lin H., Clauser K., Komuves L., Quertermous T., Conley P.B., Phillips D.R., Hart M.J. Platelet endothelial aggregation receptor 1 (PEAR1), a novel epidermal growth factor repeat-containing transmembrane receptor, participates in platelet contact-induced activation. J. Biol. Chem. 2005;280:24680–24689. doi: 10.1074/jbc.M413411200. [DOI] [PubMed] [Google Scholar]
- 12.Kardeby C., Fälker K., Haining E.J., Criel M., Lindkvist M., Barroso R., Påhlsson P., Ljungberg L.U., Tengdelius M., Rainger G.E., et al. Synthetic Glycopolymers and Natural Fucoidans Cause Human Platelet Aggregation via PEAR1 and GPIbα. Blood Adv. 2019;3:275–287. doi: 10.1182/bloodadvances.2018024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W.Y., Petit T., Cauwenberghs N., Zhang Z.Y., Sheng C.S., Thijs L., Salvi E., Izzi B., Vandenbriele C., Wei F.-F., et al. PEAR1 is not a major susceptibility gene for cardiovascular disease in a Flemish population. BMC Med. Genet. 2017;18:45. doi: 10.1186/s12881-017-0411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kauskot A., Di Michele M., Loyen S., Freson K., Verhamme P., Hoylaerts M.F. A Novel Mechanism of Sustained Platelet αIIbβ3 Activation via PEAR1. Blood. 2012;119:4056–4065. doi: 10.1182/blood-2011-11-392787. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Chen W., Lin Y., Meng X., Chen G., Wang Z., Wu J., Wang D., Li J., Cao Y., et al. Ticagrelor plus aspirin versus clopidogrel plus aspirin for platelet reactivity in patients with minor stroke or transient ischaemic attack: Open label, blinded endpoint, randomised controlled phase II trial. BMJ. 2019;365:l2211. doi: 10.1136/bmj.l2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker R.C., Bassand J.P., Budaj A., Wojdyla D.M., James S.K., Cornel J.H., French J., Held C., Horrow J., Husted S., et al. Bleeding Complications with the P2Y12 Receptor Antagonists Clopidogrel and Ticagrelor in the PLATelet Inhibition and Patient Outcomes (PLATO) Trial. Eur. Heart J. 2011;32:2933–2944. doi: 10.1093/eurheartj/ehr422. [DOI] [PubMed] [Google Scholar]
- 17.Johnson A.D., Yanek L.R., Chen M.H., Faraday N., Larson M.G., Tofler G., Lin S.J., Kraja A.T., Province M.A., Yang Q., et al. Genome-wide meta-analyses identifies seven loci associated with platelet aggregation in response to agonists. Nat. Genet. 2010;42:608–613. doi: 10.1038/ng.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qayyum R., Becker L.C., Becker D.M., Faraday N., Yanek L.R., Leal S.M., Shaw C., Mathias R., Suktitipat B., Bray P.F. Genome-wide association study of platelet aggregation in African Americans. BMC Genet. 2015;16:015–0217. doi: 10.1186/s12863-015-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keramati A.R., Yanek L.R., Iyer K., Taub M.A., Ruczinski I., Becker D.M., Becker L.C., Faraday N., Mathias R.A. Targeted deep sequencing of the PEAR1 locus for platelet aggregation in European and African American families. Platelets. 2019;30:380–386. doi: 10.1080/09537104.2018.1447659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis J.P., Backman J.D., Reny J.L., Bergmeijer T.O., Mitchell B.D., Ritchie M.D., Déry J.-P., Pakyz R.E., Gong L., Ryan K., et al. Pharmacogenomic polygenic response score predicts ischaemic events and cardiovascular mortality in clopidogrel-treated patients. Eur. Heart J. Cardiovasc. Pharmacother. 2020;6:203–210. doi: 10.1093/ehjcvp/pvz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M., Hu Y., Wen Z., Li H., Hu X., Zhang Y., Zhang Z., Xiao J., Tang J., Chen X. Association of PEAR1 rs12041331 polymorphism and pharmacodynamics of ticagrelor in healthy Chinese volunteers. Xenobiotica. 2017;47:1130–1138. doi: 10.1080/00498254.2016.1271962. [DOI] [PubMed] [Google Scholar]
- 22.Izzi B., Gianfagna F., Yang W.Y., Cludts K., De Curtis A., Verhamme P., Di Castelnuovo A., Cerletti C., Donati M.B., de Gaetano G., et al. Variation of PEAR1 DNA methylation influences platelet and leukocyte function. Clin. Epigenet. 2019;11:151. doi: 10.1186/s13148-019-0744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izzi B., Pistoni M., Cludts K., Akkor P., Lambrechts D., Verfaillie C., Verhamme P., Freson K., Hoylaerts M.F. Allele-specific DNA methylation reinforces PEAR1 enhancer activity. Blood. 2016;128:1003–1012. doi: 10.1182/blood-2015-11-682153. [DOI] [PubMed] [Google Scholar]
- 24.Faraday N., Yanek L.R., Yang X.P., Mathias R., Herrera-Galeano J.E., Suktitipat B., Qayyum R., Johnson A.D., Chen M.-H., Tofler G.H., et al. Identification of a specific intronic PEAR1 gene variant associated with greater. Blood. 2011;118:3367–3375. doi: 10.1182/blood-2010-11-320788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyack P.L., Calambokidis J., Friedlaender A., Goldbogen J., Southall B. Formal comment on Schorr GS, Falcone EA, Moretti DJ, Andrews RD (2014) First long-term behavioral records from cuvier’s beaked whales (Ziphius cavirostris) reveal record-breaking dives. PLoS ONE 9 (3): e92633. https://doi.org/10.1371/journal. pone. 0092633. PLoS ONE. 2015;10:0142287. doi: 10.1371/journal.pone.0142287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikonnikova A., Anisimova A., Galkin S., Gunchenko A., Abdukhalikova Z., Filippova M., Surzhikov S., Selyaeva L., Shershov V., Zasedatelev A., et al. Genetic Association Study and Machine Learning to Investigate Differences in Platelet Reactivity in Patients With Acute Ischemic Stroke Treated With Aspirin. Biomedicines. 2022;10:2564. doi: 10.3390/biomedicines10102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Würtz M., Nissen P.H., Grove E.L., Kristensen S.D., Hvas A.M. Genetic Determinants of On-Aspirin Platelet Reactivity: Focus on the Influence of PEAR1. PLoS ONE. 2014;9:e111816. doi: 10.1371/journal.pone.0111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faraday N., Yanek L.R., Mathias R., Herrera-Galeano J.E., Vaidya D., Moy T.F., Fallin M.D., Wilson A.F., Bray P.F., Becker L.C., et al. Heritability of platelet responsiveness to aspirin in activation pathways directly and indirectly related to cyclooxygenase-1. Circulation. 2007;115:2490–2496. doi: 10.1161/CIRCULATIONAHA.106.667584. [DOI] [PubMed] [Google Scholar]
- 29.Hu X., Liu C., Zhang M., Zhang W. Impact of the PEAR1 Polymorphism on Clinical Outcomes in Chinese Patients Receiving Dual Antiplatelet Therapy After Percutaneous Coronary Intervention. Pharmacogenomics. 2022;23:639–648. doi: 10.2217/pgs-2022-0033. [DOI] [PubMed] [Google Scholar]
- 30.Duconge J., Santiago E., Hernandez-Suarez D.F., Moneró M., López-Reyes A., Rosario M., Renta J.Y., González P., Fernández-Morales L.I., Vélez-Figueroa L.A., et al. Pharmacogenomic polygenic risk score for clopidogrel responsiveness among Caribbean Hispanics: A candidate gene approach. Clin. Transl. Sci. 2021;14:2254–2266. doi: 10.1111/cts.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Q., Cui Y., Zhao X., Zhao N. Identification of PEAR1 SNPs and Their Influences on the Variation in Prasugrel Pharmacodynamics. Pharmacogenomics. 2013;14:1179–1189. doi: 10.2217/pgs.13.108. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y., Suktitipat B., Yanek L.R., Faraday N., Wilson A.F., Becker D.M., Becker L.C., Mathias R.A. Targeted deep resequencing identifies coding variants in the PEAR1 gene that play a role in platelet aggregation. PLoS ONE. 2013;8:e64179. doi: 10.1371/journal.pone.0064179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S., Zhu J., Li H., Wang L., Niu J., Zhu B., He L., Shen L., Qin S., Fang S. Study of the Association of PEAR1, P2Y12, and UGT2A1 Polymorphisms with Platelet reactivity in response to dual antiplatelet therapy in Chinese patients. Cardiology. 2018;140:21–29. doi: 10.1159/000488101. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X., Li S., Zhao Y., Tang N., Jia T., Zhou P., Liu J., Shi L., Lu C.Y., Nie X. Genetic variants of PEAR1 and ischemic clinical outcomes in coronary artery disease patients: A systematic review and meta-analysis. Pharmacogenomics. 2021;22:641–648. doi: 10.2217/pgs-2021-0022. [DOI] [PubMed] [Google Scholar]
- 35.Lewis J.P., Ryan K., O’Connell J.R., Horenstein R.B., Damcott C.M., Gibson Q., Pollin T.I., Mitchell B.D., Beitelshees A.L., Pakzy R., et al. Genetic variation in PEAR1 is associated with platelet aggregation and cardiovascular outcomes. Circ. Cardiovasc. Genet. 2013;6:184–192. doi: 10.1161/CIRCGENETICS.111.964627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis J.P., Riaz M., Xie S., Polekhina G., Wolfe R., Nelson M., Tonkin A.M., Reid C.M., Murray A.M., McNeil J.J., et al. Genetic Variation in PEAR1, Cardiovascular Outcomes and Effects of Aspirin in a healthy elderly population. Clin. Pharmacol. Ther. 2020;108:1289–1298. doi: 10.1002/cpt.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Y., Tang X.F., He C., Song Y., Xu J.J., Meng X.M., Xu B., Gao R.L., Yuan J.Q. Effect of PEAR1 Genetic Variants on 1-Year Outcomes in Chinese Patients with acute myocardial infarction after percutaneous coronary intervention. J. Atheroscler. Thromb. 2018;25:454–459. doi: 10.5551/jat.39982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baturina O., Andreev D., Fedina L., Mirzaev K., Ivashchenko D., Ryzhikova K., Grishina E., Bochkov P., Shevchenko R., Sychev D. Influence of Clinically Significant Genes on Antiplatelet Effect of Clopidogrel and clinical outcomes in patients with acute coronary syndrome and atrial fibrillation. Pharmacology. 2022;107:216–226. doi: 10.1159/000521531. [DOI] [PubMed] [Google Scholar]
- 39.Li Z., Jiang H., Ding Y., Zhang D., Zhang X., Xue J., Ma R., Hu L., Yue Y. Platelet Endothelial Aggregation Receptor 1 Polymorphism Is Associated with Functional Outcome in Small-Artery Occlusion Stroke Patients Treated with Aspirin. Front. Cardiovasc. Med. 2021;8:664012. doi: 10.3389/fcvm.2021.664012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.