Figure 6.
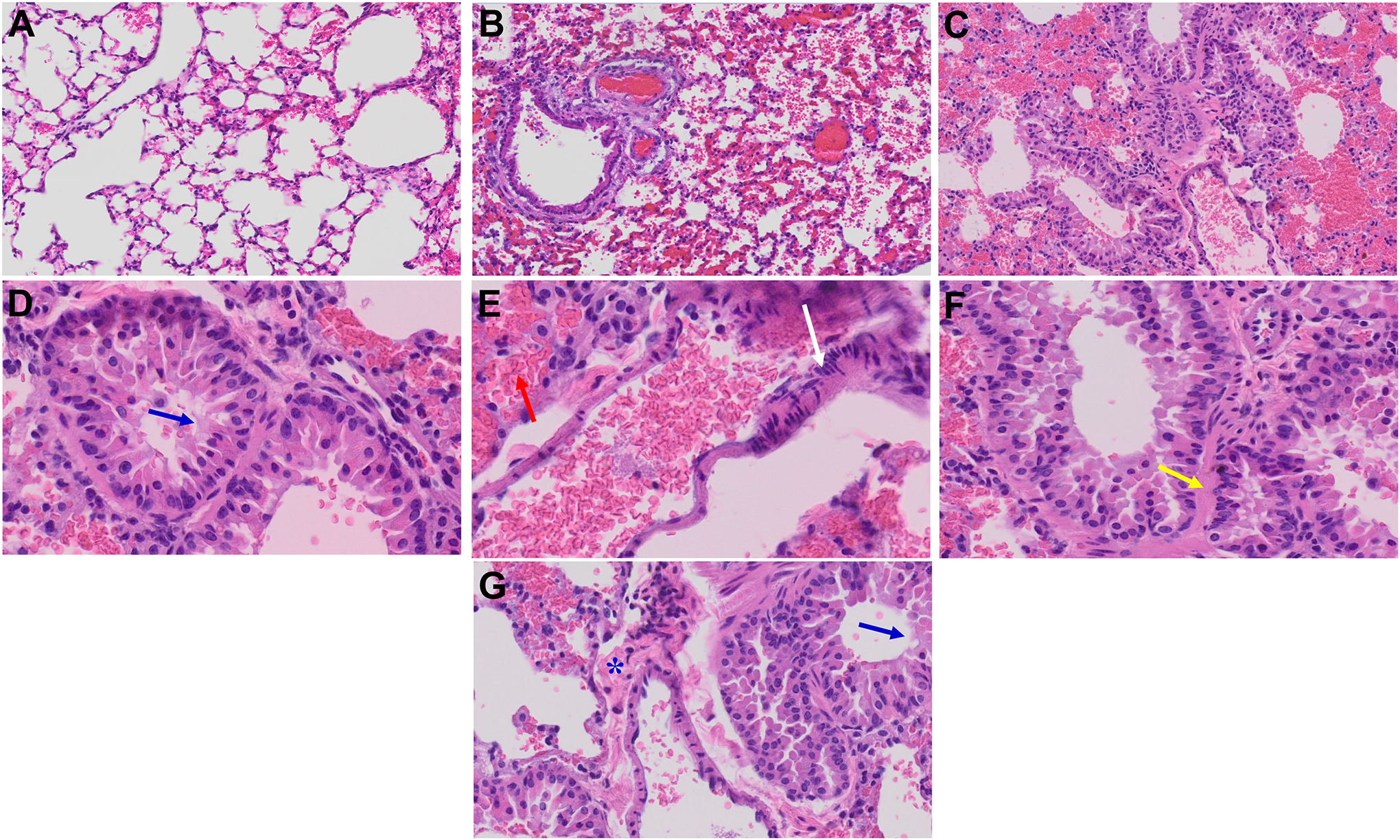
Acute and long-term changes in lung post-MHV-1 coronavirus infection. (A) Normal mouse lung. (B) Representative image from MHV-1-infected mouse lung showed “inflammation (i.e., granular degeneration of cells, and migration of leukocytes into the lungs), along with proteinaceous debris filling of the alveolar spaces with fibrillar to granular eosinophilic protein strands caused by the progressive breakdown of the capillary wall and epithelial integrity, permitting leakage of protein-rich edematous fluid into the alveoli, and the presence of hemosiderin-laden macrophages (indicating pulmonary congestion with dilated capillaries and leakage of blood into alveolar spaces). Furthermore, peribronchiolar interstitial infiltration, bronchiole epithelial cell necrosis, necrotic cell debris within alveolar lumens, alveolar exudation, hyaline membrane formation, alveolar hemorrhage with red blood cells within the alveolar space, and interstitial edema, characteristic features of infected lungs in humans with SARS-CoV-2 infection are observed in MHV-1-infected mice at acute phase (at 7 days)” (Paidas et al., 2021). (C) MHV-1-infected mouse lung (12 months post-infection). (D-G) Enlarged images of (C). Blue arrows, airspaces of alveolar ducts, and alveoli are lined by hyaline membranes; yellow arrow pulmonary edema located in bronchiolar and alveolar airspaces, along with congestion of capillaries in the septal wall, and in the perivascular interstitial spaces; white arrow, nuclear atypia and lack of polarity. Asterisks, intraluminal fibrosis. (H&E, original magnification 400 × (A-C), and (D-G) are enlarged images of (C))
