Abstract
Populations of Fusarium oxysporum f. sp. albedinis, the causal agent of Bayoud disease of date palm, are derivatives of a single clonal lineage and exhibit very similar Fot 1 hybridization patterns. In order to develop a sensitive diagnostic tool for F. oxysporum f. sp. albedinis detection, we isolated several DNA clones containing a copy of the transposable element Fot 1 from a genomic library of the date palm pathogen. Regions flanking the insertion sites were sequenced, and these sequences were used to design PCR primers that amplify the DNA regions at several Fot 1 insertion sites. When tested on a large sample of Fusarium isolates, including 286 F. oxysporum f. sp. albedinis isolates, 17 other special forms, nonpathogenic F. oxysporum isolates from palm grove soils, and 8 other Fusarium species, the primer pair TL3-FOA28 allowed amplification of a 400-bp fragment found only in F. oxysporum f. sp. albedinis. Sequence analysis showed that one of the Fot 1 copies was truncated, lacking 182 bp at its 3′ terminus. The primer pair BI03-FOA1 amplified a 204-bp fragment which overlapped the Fot 1 truncated copy and its 3′ site of insertion in the F. oxysporum f. sp. albedinis genome and identified 95% of the isolates. The primer pairs BIO3-FOA1 and TL3-FOA28 used in PCR assays thus provide a useful diagnostic tool for F. oxysporum f. sp. albedinis isolates.
Fusarium oxysporum is a common soilborne fungus which lacks a known sexual stage. Strains of F. oxysporum cause vascular wilt disease in many agricultural crops and have been classified into special forms based on their host specificity (1). F. oxysporum f. sp. albedinis (Killian and Maire) Gordon is the causal agent of Bayoud disease, a devastating disease of date palm (Phoenix dactylifera L.) that only occurs in Algerian and Moroccan oases (13). The pathogen is easily spread by exchange of contaminated material; at present, strict phytosanitary rules are applied at borders of date palm-growing countries that are free of Bayoud disease. Detection and identification of F. oxysporum f. sp. albedinis remain difficult, mainly because time-consuming inoculation tests are required to assess pathogenicity.
We previously showed that F. oxysporum f. sp. albedinis isolates are genetically closely related and belong to a single clonal lineage (15, 22). Genomic analyses also have shown that the fungus carries repetitive DNA sequences homologous to the DNA transposable element Fot 1, first cloned from F. oxysporum f. sp. melonis and widely distributed in F. oxysporum (3). Depending on the strain, 15 to 26 EcoRI fragments hybridizing with the Fot 1 sequence were detected, and several were conserved (15, 22). Fot 1 hybridization patterns of F. oxysporum f. sp. albedinis and other, nonpathogenic F. oxysporum isolates from palm grove soils were very different, with the nonpathogens exhibiting only zero to eight EcoRI hybridizing bands (21).
We hypothesized that at least one Fot 1 copy is inserted at a genomic location specific for F. oxysporum f. sp. albedinis. If this hypothesis is correct, this region could be amplified by PCR of F. oxysporum f. sp. albedinis DNA with primers overlapping the 3′ or 5′ end of the Fot 1 copy and its genomic region of insertion.
Testing this hypothesis requires (i) cloning of the Fot 1 copies present in the genome of the pathogen, together with their flanking genomic sequences, (ii) sequencing of the 3′ and 5′ insertion regions and designing of primers for PCR amplification, and (iii) testing for amplification of a specific DNA fragment in F. oxysporum f. sp. albedinis isolates.
In this report, we describe the cloning of several Fot 1 copies from F. oxysporum f. sp. albedinis, the characterization of a truncated copy, and the subsequent development of specific oligonucleotides to use as primers in PCR assays for quickly identifying this pathogen.
MATERIALS AND METHODS
Fungal isolates.
The isolates examined are listed in Table 1. The F. oxysporum f. sp. albedinis, F. oxysporum, and F. moniliforme isolates were collected from different oases in Algeria and Morocco. The F. oxysporum f. sp. elaeidis (6, 14) and F. oxysporum f. sp. vasinfectum (7) isolates belonged to several vegetative compatibility groups and had different molecular haplotypes. The other Fusarium strains examined were obtained either as isolates from the Laboratoire de la Flore Pathogène des Sols (Institut National de la Recherche Agronomique, Dijon, France) or as DNA samples from H. C. Kistler (University of Florida, Gainesville) or S. Bentley (University of Queensland, Brisbane, Queensland, Australia). All cultures were single spore and were maintained in glycerol under liquid nitrogen until use or as mycelia on potato dextrose agar slants.
TABLE 1.
Fusarium species tested and results of PCR amplification with the primer pairs designed in this study
| Fusarium species | No. of isolates | Source | Geographic origin | PCR resulta with:
|
|
|---|---|---|---|---|---|
| BIO3-FOA1 | TL3-FOA28 | ||||
| F. oxysporum f. sp. albedinis | 208 | Date palm leaf | Morocco | 198+/10− | + |
| 3 | Date palm root | Morocco | + | + | |
| 5 | Palm grove soil | Morocco | + | + | |
| 70 | Date palm leaf | Algeria | 64+/6− | + | |
| F. oxysporum | 28 | Palm grove soil | Algeria | − | − |
| 26 | Palm grove soil | Morocco | − | − | |
| F. oxysporum f. sp. basilicum | 1 | Basil | Australia | − | − |
| F. oxysporum f. sp. canariensis | 17 | Ornamental palm | Diverseb | − | − |
| F. oxysporum f. sp. cicerii | 1 | Chickpea | Morocco | − | − |
| F. oxysporum f. sp. conglutinans | 1 | Cabbage | United States | − | − |
| F. oxysporum f. sp. cubense | 1 | Banana | − | − | |
| F. oxysporum f. sp. dianthi | 1 | Carnation | Australia | − | − |
| F. oxysporum f. sp. elaeidis | 6 | Oil palm | Diversec | − | − |
| F. oxysporum f. sp. lycopersici | 2 | Tomato | Morocco and United States | − | − |
| F. oxysporum f. sp. matthioli | 1 | Matthiola | United States | − | − |
| F. oxysporum f. sp. medicaginis | 1 | Alfalfa | Australia | − | − |
| F. oxysporum f. sp. melonis | 1 | Muskmelon | France | − | − |
| F. oxysporum f. sp. niveum | 1 | Watermelon | Australia | − | − |
| F. oxysporum f. sp. pisi | 2 | Pea | Australia and United States | − | − |
| F. oxysporum f. sp. raphani | 1 | Radish | United States | − | − |
| F. oxysporum f. sp. vasinfectum | 6 | Cotton | Diversed | − | − |
| F. oxysporum f. sp. zingiberi | 1 | Ginger | Australia | − | − |
| F. arthrosporoides | 1 | France | − | − | |
| F. avanaceum | 1 | France | − | − | |
| F. culmorum | 1 | France | − | − | |
| F. graminearum | 1 | France | − | − | |
| F. lateritium | 1 | France | − | − | |
| F. moniliforme | 7 | Palm grove soil | Morocco | − | − |
| F. roseum | 1 | France | − | − | |
| F. scirpii | 1 | France | − | − | |
| F. trincitum | 1 | France | − | − | |
+, amplification of a DNA fragment; −, no amplification of a DNA fragment.
Canary Islands, France, Italy, Japan, and the United States.
Brazil, Ivory Coast, Ecuador, Ghana, and Zaire.
Brazil, Egypt, India, Sudan, and the United States.
Cloning of the Fot 1 copies.
Genomic DNA from F. oxysporum f. sp. albedinis GH3 (International Mycological Institute [IMI] accession number 377827) (isolated in Ghardaia, Algeria, in 1993) was isolated and purified by cesium chloride gradient centrifugation as described previously (2). Purified DNA (380 ng) was digested to completion with the restriction enzyme ClaI (Boehringer Mannheim Biochemicals, Meylan, France) and ligated into ClaI-restricted pBluescript (500 ng) with a ligation kit (Amersham, Les Ulis, France). Escherichia coli DH5α competent cells were prepared and transformed with the ligation mixture (20 μl) following the procedure of Inoue et al. (11). Transformed bacterial colonies grown on Luria broth-ampicillin (100 μg ml−1) plates were transferred to nylon membranes (Nylon N; Amersham) and lysed by standard procedures (17). DNA bound to nylon filters was hybridized with 32P-labeled Fot 1 to detect plasmids containing Fot 1.
Characterization of recombinant plasmids.
Transformed E. coli was grown in Luria broth amended with ampicillin (100 μg ml−1), and plasmid DNA was isolated with a minipreparation alkaline lysis protocol (17). Genomic insert sizes were determined by digestion with ClaI followed by electrophoresis in an 0.8% agarose gel. DNA fragments were blotted onto Nylon N membranes by alkaline vacuum transfer (TE 80 TransVac; Hoefer Scientific Instruments, San Francisco, Calif.) and hybridized with radioactively labeled Fot 1.
Sequencing reactions.
Inserts were sequenced either by automated sequencing (Applied Biosystems, Inc.) with TaqFS polymerase (dye deoxy terminator kit; Perkin-Elmer Cetus, Saint-Quentin en Yvelines, France) or by manual sequencing with 35S-dATP and T7 polymerase (T7 sequencing kit; Pharmacia LKB Biotechnology, Orsay, France). 35S-labeled dATP reaction products were separated by 7% polyacrylamide–urea gel electrophoresis and identified by autoradiography. DNA sequences flanking Fot 1 insertion sites in plasmid clones were determined by extension of primers TL3 and BIO3 (Table 2). Primers were synthesized based on the sequence of Fot 1 (3): TL3 is located 179 bp from the Fot 1 5′ end, and BIO3 is located 290 bp from the Fot 1 3′ end.
TABLE 2.
Sequences of specific primers used for detecting F. oxysporum f. sp. albedinis
| Target clone | Primer | Sequence | Fot 1 matching region | Amplicon size (bp) |
|---|---|---|---|---|
| pFalb11 | FOA1 | CAGTTTATTAGAAATGCCGCC | 3′ flanking | 204 |
| BIO3 | GGCGATCTTGATTGTATTGTGGTG | 3′ end | ||
| pFalb28 | FOA28 | ATCCCCGTAAAGCCCTGAAGC | 5′ flanking | 400 |
| TL3 | GGTCGTCCGCAGAGTATACCGGC | 5′ end |
Fusarium sp. PCR analysis.
Fungal DNA extraction was performed as previously described (7) and with the rapid NaOH procedure described elsewhere (9). The 3′ insertion site of the Fot 1 copy in plasmid pFalb11 and the 5′ insertion site of the Fot 1 copy in plasmid pFalb28 were amplified with primer pairs BIO3-FOA1 and TL3-FOA28, respectively (Table 2). Each reaction mixture contained 1.5 mM MgCl2; 100 μM (each) dATP, dCTP, dGTP, and dTTP; 1 μM (each) primers BIO3 and FOA1 or 0.5 μM (each) primers TL3 and FOA28; 25 ng of genomic DNA; 2.5 μl (or 1.25 μl) of 10×-concentrated reaction buffer; and 0.5 U (or 0.25 U) of DNA polymerase (Goldstar DNA polymerase; Eurogentec, Seraing, Belgium). Negative controls (no DNA target) were included in every experiment to test for contamination. Amplifications were performed with a DNA thermal cycler (PHC-3 [Techne] or Thermojet [Eurogentec]) programmed as follows: 1 cycle for 4 min at 95°C followed by 30 cycles for 30 s at 92°C, 30 s at 60°C, and 30 s at 72°C for the BIO3-FOA1 primer pair and 30 cycles for 30 s at 92°C, 30 s at 62°C, and 45 s at 72°C for the TL3-FOA28 primer pair. One cycle for 15 min at 72°C was conducted after the 30 cycles. After amplification, 8 μl of the reaction mixture was loaded onto a 1.4% agarose gel, separated by electrophoresis, stained with ethidium bromide, and photographed under UV light.
Fot 1 amplification, probe preparation, and hybridization conditions. Fot 1 was amplified either from F. oxysporum f. sp. albedinis isolates or from the 1.9-kb Fot 1 sequence provided by Daboussi et al. (3). The primer LTR (5′ CCCATGTAACCGACCCCCCCTGG 3′) homologous to the Fot 1 inverted terminal repeats was used for PCR with the following conditions: 1 cycle for 4 min at 95°C followed by 30 cycles for 1.5 min at 92°C, 1 min at 60°C, and 1.5 min at 72°C. The amplified fragment was recovered by ethanol precipitation for probe preparation. One hundred nanograms of the purified probe was labeled with 20 μCi of [32P]dCTP (Amersham) by random priming according to the manufacturer’s specifications (T7 Quick Primer kit; Pharmacia LKB Biotechnology). Membrane-bound DNA was hybridized to the labeled probe at 65°C overnight in 15 ml of buffer (hybridization tablets buffer; Amersham) and washed for 20 min in 50 ml of 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% (wt/vol) sodium dodecyl sulfate (SDS) at room temperature, 15 min in 50 ml of 1× SSC–0.1% SDS at 65°C, and 15 min in 50 ml of 0.5× SSC–0.1% SDS at 65°C. Hybridization signals were detected by autoradiography at −80°C.
Nucleotide sequence accession numbers.
The sequences of the flanking regions contained in clones pFalb11 and pFalb28 were deposited at GenBank (accession numbers AF033099 and AF033098).
RESULTS
Choice of reference strain and restriction enzyme for cloning of the Fot 1 copies.
We digested F. oxysporum f. sp. albedinis DNA with four restriction enzymes (ClaI, EcoRI, XbaI, and XhoI) that did not cut in the 1,928-bp Fot 1 sequence from F. oxysporum f. sp. melonis (3). We found that the F. oxysporum f. sp. albedinis Fot 1 sequence contains an EcoRI site not present in the F. oxysporum f. sp. melonis Fot 1 sequence. We thus used ClaI to construct a genomic library from F. oxysporum f. sp. albedinis GH3. This strain carries approximately 14 copies of Fot 1, and most of the resulting ClaI fragments were less than 8 kb long and could be cloned into pBluescript.
Cloning of the Fot 1 copies and sequencing of flanking regions.
We identified 42 plasmids that could hybridize with Fot 1; 18 of them carried an F. oxysporum f. sp. albedinis DNA fragment which could be excised from the plasmid by digestion with ClaI. The 3′ and 5′ Fot 1 insertion sites were obtained for four clones: pFalb11 (insert size, 2.8 kb), pFalb17 (9 kb), pFalb20 (6 kb), and pFalb28 (6.5 kb). With clones pFalb17, pFalb20, and pFalb28, only the 5′ Fot 1-flanking sequence was obtained, because these plasmids had a ClaI site 70 bp from the Fot 1 3′ end, thus preventing sequencing of the flanking sequence. Oligonucleotides to use as PCR primers were deduced from the 5′ Fot 1-flanking sequence of all four clones and from the 3′ Fot 1-flanking sequence of pFalb11 and were tested in combination with TL3 and BIO3, respectively. PCR amplifications of the genomic DNA of F. oxysporum f. sp. albedinis GH3 were obtained with primer pairs TL3-FOA28 and BIO3-FOA1.
PCR assays for specific identification of F. oxysporum f. sp. albedinis strains.
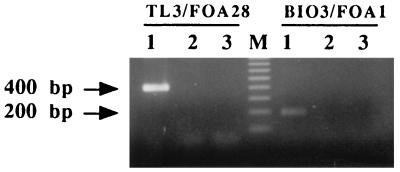
Primer pairs TL3-FOA28 and BIO3-FOA1 were tested on DNA extracted from the Fusarium isolates listed in Table 1, including 286 F. oxysporum f. sp. albedinis isolates, 17 other special forms, nonpathogenic F. oxysporum isolates from palm grove soils, and 8 other Fusarium species. Primer pair TL3-FOA28 could be used to amplify a 400-bp DNA fragment for all of the F. oxysporum f. sp. albedinis isolates tested, and primer pair BIO3-FOA1 could be used to amplify a 204-bp DNA fragment for only 95% (270 of 286) of the F. oxysporum f. sp. albedinis isolates (Fig. 1). No amplification was observed when the DNA template originated from the other Fusarium isolates (Fig. 1 and Table 1). When various amounts of DNA from strain GH3 were tested, less than 100 pg of purified F. oxysporum f. sp. albedinis DNA could be efficiently detected with primer pairs TL3-FOA28 and BIO3-FOA1.
FIG. 1.
Ethidium bromide-stained 1.4% agarose gel showing results of PCR experiments with primer pairs TL3-FOA28 and BIO3-FOA1. Lanes: 1 to 3, amplification products from F. oxysporum f. sp. albedinis isolates, other F. oxysporum isolates, and a negative control, respectively; M, molecular size markers (100-bp ladder; Eurogentec). Fragment sizes are indicated on the left. Note the selective amplification of a 400- or 200-bp fragment from F. oxysporum f. sp. albedinis isolates only.
Characterization of a 3′-truncated Fot 1 copy.
The Fot 1 element carried by clone pFalb11 lacked 182 bp at its 3′ end in comparison with the original Fot 1 sequence (3). With primer TL3, up to 410 bp of the 5′ part of the insert was sequenced; 180 bp of this sequence was homologous to the Fot 1 sequence (positions 1 to 180). Primer BIO3 allowed 235 bp of clone pFalb11 to be read (Fig. 2); the first 75 bp of this sequence was identical to Fot 1 (positions 1671 to 1746), and the additional 160 bp of this sequence was very different, indicating that pFalb11 apparently carries a 3′-truncated Fot 1 copy. The 160-bp flanking region of the truncated copy did not show similarity with the sequences listed in the GenBank or EMBL data banks. PCR tests with primer pair BIO3-FOA1 showed that the truncated copy seemed to be present in the genomes of 95% of the F. oxysporum f. sp. albedinis strains tested (Table 1).
FIG. 2.
Nucleotide sequence of the 3′ region of pFalb11 aligned with the 1,928-bp Fot 1 sequence (Fom24) from F. oxysporum f. sp. melonis (3). The sequence was obtained with BIO3 as a sequencing primer (underlined). The position of the deduced PCR primer FOA1 is underlined. Asterisks indicate conserved nucleotides, and blank areas indicate missing data.
DISCUSSION
The transposable element Fot 1 is widely distributed in the species F. oxysporum but displays a discontinuous pattern of conservation in the special forms (4). The aim of this study was to identify copies of the transposable element Fot 1 inserted at specific loci within the genome of F. oxysporum f. sp. albedinis isolates and to use them as templates for the PCR characterization of F. oxysporum f. sp. albedinis DNA.
We characterized two Fot 1 copies, carried by plasmids pFalb28 and pFalb11. Two primers, FOA28 and FOA1, specific for genomic sequences where the Fot 1 copies were inserted, enabled the amplification of F. oxysporum f. sp. albedinis DNAs only when paired with Fot 1-specific primers TL3 and BIO3, respectively. These genomic loci are probably common to all F. oxysporum isolates, while the presence of Fot 1 at these loci seems to be F. oxysporum f. sp. albedinis specific.
Primer pair TL3-FOA28 could be used to amplify a 400-bp fragment from all of the F. oxysporum f. sp. albedinis isolates. With primer pair BIO3-FOA1, a 204-bp fragment could be amplified from 95% of these isolates. Sixteen F. oxysporum f. sp. albedinis strains were not amplified with primer pair BIO3-FOA1. Additional hybridization experiments indicated that none of these 16 isolates carried a Fot 1 insertion (truncated or full length) at the corresponding locus.
In this study, we showed that the Fot 1 copy in pFalb11 is truncated, lacking 182 bp at its 3′ end, and that this truncated copy was carried by 95% of the F. oxysporum f. sp. albedinis isolates tested. Given the high percentage of Algerian and Moroccan strains that possess the truncated copy and epidemiological data indicating that the Algerian oases were contaminated from Morocco, we think it likely that an ancestral F. oxysporum f. sp. albedinis strain had a full-length copy that became truncated in one sublineage and was fully lost in another.
We have thus succeeded in obtaining specific oligonucleotides for identifying the date palm pathogen F. oxysporum f. sp. albedinis. PCR has been successfully used as a sensitive and powerful diagnostic tool for microorganisms in the environment and has been applied to the detection of phytopathogenic and symbiotic fungi in plants or soil (5, 9, 10, 18, 20, 23). This work reports a novel development for a PCR to accurately assign F. oxysporum strains to a particular special form. Our study shows the usefulness of transposons and their flanking regions for designing PCR primers specific for the F. oxysporum special forms. We have taken advantage of the high level of genetic relatedness among isolates pathogenic for date palm to develop a sensitive PCR assay. Less than 10−10 g of purified DNA could be efficiently detected, and further studies were carried out to detect the pathogen directly in host tissues.
This approach differs slightly from approaches that successfully developed PCR primers to identify F. oxysporum pathogenic isolates (16, 24). Unless F. oxysporum f. sp. albedinis shows a remarkable level of genetic homogeneity compared to most of the F. oxysporum special forms studied (8, 12, 22), the same strategy could be used for other special forms carrying Fot 1. In the same way, in fungal species where several transposable elements have been characterized, such as Magnaporthe grisea (19), this transposon-based approach could be used for developing diagnostic PCR tests. Recent characterizations of microsatellite loci in fungi also have proved to be useful for obtaining primers derived from the flanking regions (9) and could provide a novel source of target sequences for PCR tests.
ACKNOWLEDGMENTS
We thank colleagues who provided us with fungal isolates or DNA and Kamel Chabane (ICARDA, Genetic Resources Unit, Aleppo, Syria) for technical assistance.
M. Ouinten was supported by the Ministère Français des Affaires Etrangères, K. Chabane was supported by ORSTOM, and A. Tantaoui was supported by the International Foundation for Science.
REFERENCES
- 1.Armstrong G M, Armstrong J K. Formae speciales and races of Fusarium oxysporum causing wilt diseases. In: Nelson P E, Toussoun T A, Cook R J, editors. Fusarium: diseases, biology and taxonomy. University Park: Pennsylvania State University Press; 1981. pp. 391–399. [Google Scholar]
- 2.Daboussi M J, Djeballi A, Gerlinger C, Blaiseau P L, Bouvier I, Cassan M, Lebrun M H, Parisot D, Brygoo Y. Transformation of seven species of filamentous fungi using the nitrate reductase gene of Aspergillus nidulans. Curr Genet. 1989;15:453–456. doi: 10.1007/BF00376803. [DOI] [PubMed] [Google Scholar]
- 3.Daboussi M J, Langin T, Brygoo Y. Fot1, a new family of fungal transposable elements. Mol Gen Genet. 1992;232:12–16. doi: 10.1007/BF00299131. [DOI] [PubMed] [Google Scholar]
- 4.Daboussi M J, Langin T. Transposable elements in the fungal plant pathogen Fusarium oxysporum. Genetica. 1994;93:49–59. [Google Scholar]
- 5.Di Bonito R, Elliott M L, Des Jardin E A. Detection of an arbuscular mycorrhizal fungus in roots of different plant species with the PCR. Appl Environ Microbiol. 1995;61:2809–2810. doi: 10.1128/aem.61.7.2809-2810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dossa C, Pando-Bahuon A, Renard J L, Boisson C. Determination of vegetative compatibility groups in African Fusarium oxysporum strains isolated from vascular wilt-infected oil palms. Oleagineux. 1991;46:145–147. [Google Scholar]
- 7.Fernandez D, Assigbetse K B, Dubois M P, Geiger J P. Molecular characterization of races and vegetative compatibility groups in Fusarium oxysporum f. sp. vasinfectum. Appl Environ Microbiol. 1994;60:4039–4046. doi: 10.1128/aem.60.11.4039-4046.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez D, Ouinten M, Tantaoui A, Geiger J-P. Molecular records of micro-evolution within the Algerian population of Fusarium oxysporum f. sp. albedinis during its spread to new oases. Eur J Plant Pathol. 1997;103:485–490. [Google Scholar]
- 9.Groppe K, Boller T. PCR assay based on a microsatellite-containing locus for detection and quantification of Epichloë endophytes in grass tissue. Appl Environ Microbiol. 1997;63:1543–1550. doi: 10.1128/aem.63.4.1543-1550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamelin R C, Berube P, Gignac M, Bourassa M. Identification of root rot fungi in nursery seedlings by nested multiplex PCR. Appl Environ Microbiol. 1996;62:4026–4031. doi: 10.1128/aem.62.11.4026-4031.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 12.Kistler H C. Genetic diversity in the plant-pathogenic fungus Fusarium oxysporum. Phytopathology. 1997;87:474–479. doi: 10.1094/PHYTO.1997.87.4.474. [DOI] [PubMed] [Google Scholar]
- 13.Louvet J, Toutain G. Bayoud, Fusarium wilt of date palm. In: Nelson P E, Toussoun T A, Cook R J, editors. Fusarium: diseases, biology and taxonomy. University Park: Pennsylvania State University Press; 1981. pp. 13–20. [Google Scholar]
- 14.Mouyna I, Renard J L, Brygoo Y. DNA polymorphism among Fusarium oxysporum f. sp. elaeidis populations from oil palm, using a repeated and dispersed sequence “Palm.”. Curr Genet. 1996;30:174–180. doi: 10.1007/s002940050117. [DOI] [PubMed] [Google Scholar]
- 15.Ouinten M. Diversité et structure génétiques des populations algériennes de Fusarium oxysporum f. sp. albedinis, agent de la fusariose vasculaire (Bayoud) du palmier dattier. Ph.D. thesis. Montpellier, France: Université Montpellier II; 1996. [Google Scholar]
- 16.Plyler T, Kistler H C, Fernandez D. Development of a PCR detection method for Fusarium oxysporum f. sp. canariensis. Phytopathology. 1997;87:S78. . (Abstract.) [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18.Schilling A G, Moller E M, Geiger H H. Polymerase chain reaction-based assays for species-specific detection of Fusarium culmorum, F. graminearum, and F. avenaceum. Phytopathology. 1996;86:515–522. [Google Scholar]
- 19.Schull V, Hamer J. Genetic differentiation in the rice blast fungus revealed by the distribution of the Fosbury retrotransposon. Fungal Genet Biol. 1996;20:59–69. doi: 10.1006/fgbi.1996.0010. [DOI] [PubMed] [Google Scholar]
- 20.Simon L, Lalonde M, Bruns T D. Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. PCR Methods Appl. 1992;2:76–80. doi: 10.1128/aem.58.1.291-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tantaoui A. Caractérisation moléculaire et diversité génétique chez Fusarium oxysporum f. sp. albedinis, responsable de la fusariose vasculaire (Bayoud) du palmier dattier. Ph.D. thesis. Marrakech, Morocco: University of Marrakech; 1994. [Google Scholar]
- 22.Tantaoui A, Ouinten M, Geiger J-P, Fernandez D. Characterization of a single clonal lineage of Fusarium oxysporum f. sp. albedinis causing Bayoud disease of date palm (Phoenix dactylifera L.) in Morocco. Phytopathology. 1996;86:787–792. [Google Scholar]
- 23.Volossiouk T, Robb E J, Nazar R N. Direct DNA extraction for PCR-mediated assays of soil organisms. Appl Environ Microbiol. 1995;61:3972–3976. doi: 10.1128/aem.61.11.3972-3976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woudt L P. Identification of Fusarium oxysporum f. sp. cyclaminis by DNA fingerprinting and PCR. Bull OEPP. 1995;25:109–112. [Google Scholar]