Table 1.
List of short-chain fatty acids.
| Number of Carbon Atoms | Common Name | Systematic Name | Molecular Formula | Structural Formula |
Mass (g/mol) | Diagram |
|---|---|---|---|---|---|---|
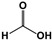
| C1 | Formic acid | Methanoic6 acid | CH2O2 | HCOOH | 46.03 |
|
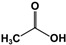
| C2 | Acetic acid | Ethanoic acid | C2H4O2 | CH3COOH | 60.05 |
|
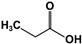
| C3 | Propionic acid | Propanoic acid | C3H6O2 | CH3CH2COOH | 74.08 |
|
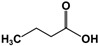
| C4 | Butyric acid | Butanoic acid | C4H8O2 | CH3(CH2)2COOH | 88.11 |
|
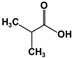
| C4 | Isobutyric acid | 2-Methyl propanoic acid | C4H8O2 | (CH3)2CHCOOH | 88.11 |
|
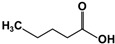
| C4 | Valeric acid | Pentanoic acid | C5H10O2 | CH3(CH2)3COOH | 102.13 |
|
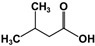
| C5 | Isovaleric acid | 3-Methylbutanoic acid | C5H10O2 | (CH3)2CHCH2COOH | 102.13 |
|
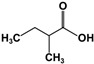
| C5 | 2-Methylbutyric acid |
2-Methylbutyric acid |
C5H10O2 | CH3CH2CH(CH3)COOH | 102.13 |
|
