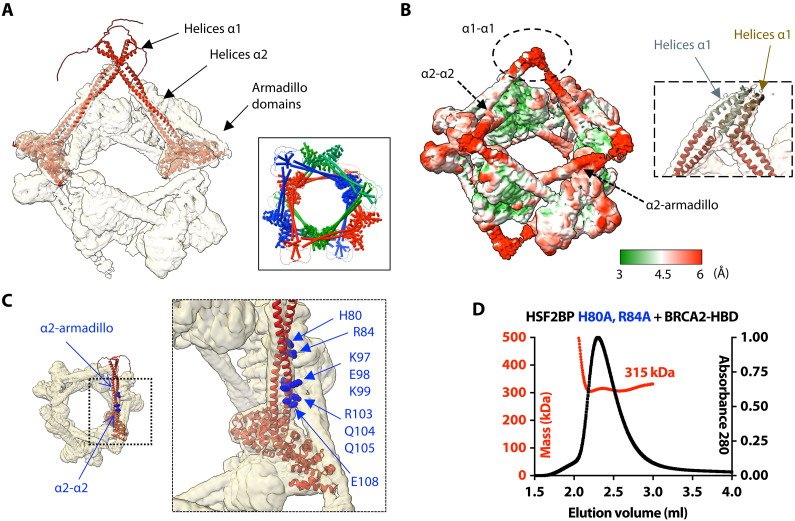
Fig. 3. The ring-shaped complex is assembled through a large set of interfaces, involving not only armadillo-BRCA2 but also α1-α1, α2-α2, and α2-armadillo contacts.
(A) Docking of two (similar) AlphaFold models of the HSF2BP dimer into the cryo-EM map. The map is displayed with an electron density threshold of 0.035. The cartoon views of the two AlphaFold models are colored in red. Each model consists of a disordered region, a short helix α1, a large helix α2, and an armadillo domain. In the boxed panel, three pairs of HSF2BP tetramers docked into the cryo-EM map are displayed in three different colors. (B) Cryo-EM map displayed with a lower electron density threshold (0.025) and colored as a function of the local resolution. The colors are from green (3 Å) to red (6 Å). A dashed oval identifies a map region corresponding to the four helices α1, whereas arrows indicate regions of α2-α2 and α2-armadillo contacts. In the inset, four helices α2 are docked as in (A) (in red), whereas four parallel helices α1 (in gray and brown) are manually positioned into the cryo-EM density displayed with an electron density threshold (0.010). (C) Position of the α2 residues mutated to test their role in the assembly and/or function of the ring-shaped complex. An AlphaFold model of HSF2BP dimer (in red) is docked into the cryo-EM map. Residues at the α2-α2 and α2-armadillo interfaces are marked with standard and bold labels, respectively. (D) SEC-MALS analysis of the mutant HSF2BP H80A-R84A bound to BRCA2-HBD (column: BIOSEC 3; see also fig. S4).