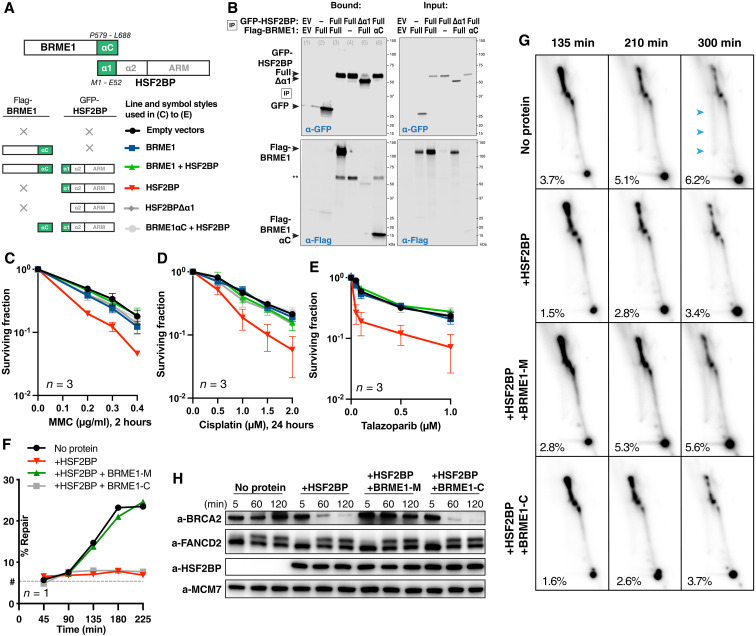
Fig. 6. BRME1 protects cancer cells from HSF2BP and prevents BRCA2 degradation during interstrand crosslink repair.
(A) Schematic of the six HSF2BP and BRME1 fragment combinations used in (B) to (E), next to the corresponding line styles; × — protein not present. Interacting α helices are shown as green blocks. (B) Co-immunoprecipitation of HSF2BP and BRME1 variants used in clonogenic survivals. Proteins eluted from the anti–green fluorescent protein (GFP) beads were detected by immunoblotting with the indicated antibodies. (C to E) Clonogenic survival of HeLa cells stably producing HSF2BP and BRME1 variants, as indicated in (A), and treated with mitomycin C (MMC), cisplatin, or talazoparib. The experiments were repeated three times, and means and SEM are plotted. (F) Efficiency of synthetic cisplatin interstrand crosslink repair in Xenopus egg extract (24) in the presence or absence of HSF2BP, the BRME1 peptides BRME1-M (binding HSF2BP), and BRME1-C (not binding HSF2BP; Fig. 4, A and B) (24). Replicate is shown in fig. S7 (C and D). (G) HR intermediate formation during the repair of synthetic cisplatin DNA interstrand crosslink in Xenopus egg extract monitored by 2D agarose gel electrophoresis in the presence or absence of HSF2BP, the BRME1 peptides BRME1-M, and BRME1-C (see Fig. 4, A and B). The X-arc that contains HR intermediates is indicated by blue arrows in the top right panel, the percentage of the signal localizing to it is indicated, as detailed previously (24), and replicate is shown in fig. S7E. (H) Effect of HSF2BP and BRME1-M or BRME1-C peptides on the endogenous Xenopus BRCA2 protein during the time course (5 to 120 min) of the interstrand crosslink repair reaction. Antibodies used for immunoblotting are indicated (24), and replicate is shown in fig. S7F.