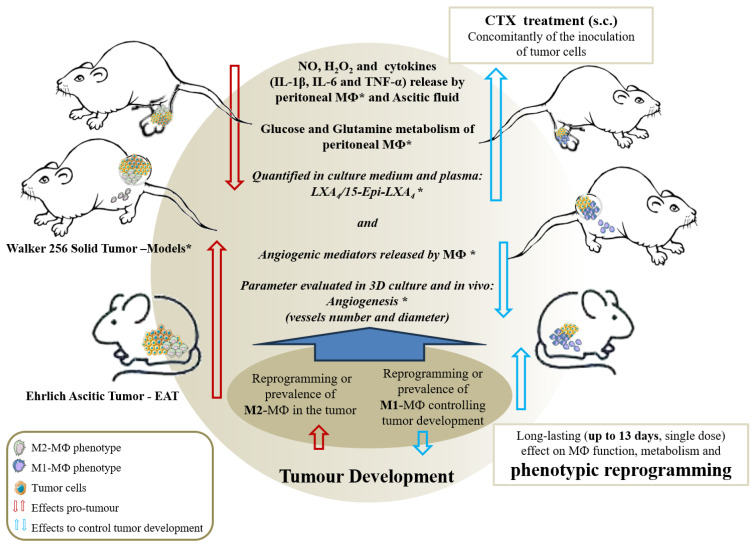
Figure 6.
Scheme proposed for the importance of phenotypic reprogramming of macrophages induced by CTX in the tumor microenvironment. The data found in the present study demonstrate that CTX induces phenotypic reprogramming of macrophages, with a prevalence of M1 macrophages in the tumor microenvironment. This prevalent M1 profile agrees with the CTX-induced modifications on the metabolism and secretory activity of peritoneal macrophages obtained from tumor-bearing animals * [23,32]. The stimulatory action on the metabolism of macrophages was characterized by an increase in the release of H2O2, the production of NO, the secretion of pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) and the increased maximum activity of hexokinase, glucose-6-phosphate dehydrogenase and citrate synthase, accompanied by an increased secretion of LXA4 and 15-Epi-LXA4 by these cells, also characterized in in vitro studies [30,36]. All these mediators lead to the inhibition of tumor development. The present study shows for the first time that CTX induces phenotypic reprogramming, explaining the metabolism and secretory activity of macrophages compatible with the M1 profile, as previously demonstrated, with long-lasting action, since they are observed up to 13 days after the administration of a single dose of toxin [37].