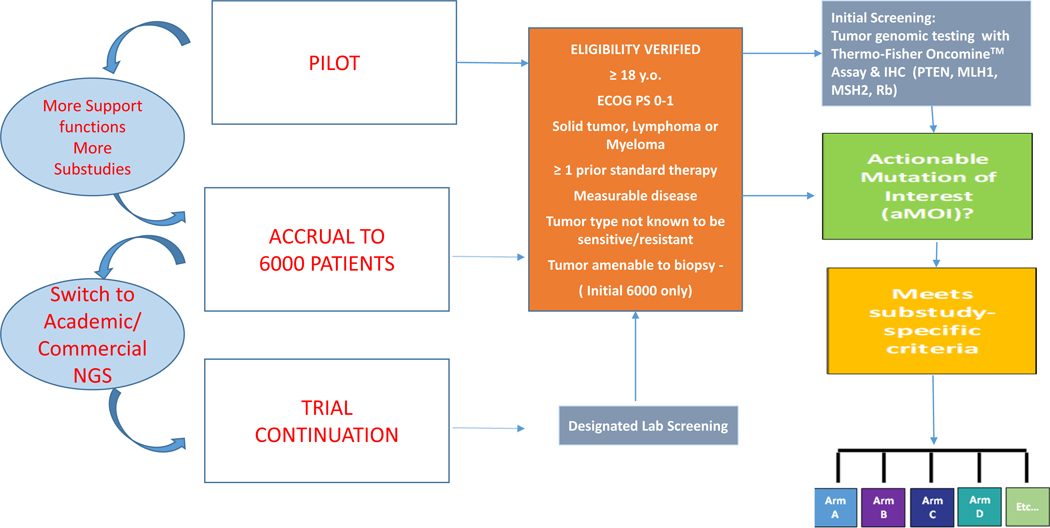
Figure 1. NCI-MATCH Platform Trial Design.
The trial was implemented in three parts, sequentially: a pilot phase (795 patients) that led to multiple modifications; a screening accrual phase (~6000 patients); and a continuation phase whereby patients were recruited from a network of academic and commercial laboratories. In the initial 6000-patient screening period, eligibility was assessed prior to obtaining a dedicated biopsy, while during the continuation period, tumor samples were assayed as part of standard clinical practice, and eligibility assessed when a candidate mutation was identified. In either case, eligible patients with qualifying tumor molecular aberrations were assigned to a therapeutic substudy, to receive treatment directed to their molecular profile. As a further quality control measure, for patients tested at designated labs, confirmation sequencing was conducted by the NCI-MATCH Central Labs and only patients whose tumor genomic profile was confirmed in this manner were included for efficacy endpoints. Each substudy was a separate Phase II trial constituting a drug-genomic driver pair. Patients were assigned to substudies with the assistance of a decision tool (MATCHBox), overseen for appropriateness by a team of medical oncologists, laboratory scientists, and bioinformaticists from the trial leadership that reviewed every patient. In each substudy, the initial aim was to accrue 35 patients, assuming 31 would be eligible and start protocol treatment (analyzable); there was provision to increase accrual to 70 in selected arms. The primary endpoint for each substudy was objective response rate (ORR), defined as rate of complete or partial response, as assessed by RECIST guidelines (12).