Abstract
Introduction
Deucravacitinib, a newly approved oral medication for the treatment of patients with moderate to severe plaque psoriasis, demonstrated efficacy versus apremilast and placebo in two phase 3 randomized controlled trials (RCTs). A systematic review and network meta-analysis (NMA) indirectly compared deucravacitinib with other relevant systemic biologic/nonbiologic treatments.
Methods
Online databases were searched for RCTs published through October 2021. Eligible studies were head-to-head comparisons between systemic therapies and/or placebo reporting 50%, 75%, 90%, or 100% improvement in Psoriasis Area and Severity Index (PASI) from baseline in adults with moderate to severe plaque psoriasis. Comparisons included tumor necrosis factor inhibitors, interleukin (IL)-17, IL-23, and IL 12/23 inhibitors, and systemic nonbiologics. A multinomial Bayesian NMA was used to derive estimates of the relative efficacy of deucravacitinib and other systemic therapies. Response probabilities for each treatment and corresponding 95% credible intervals (CrIs) for achieving a PASI response were calculated over short-, mid-, and long-term follow-up (weeks 10–16, 24–28, and 44–60).
Results
The NMA included 47 RCTs. Deucravacitinib showed the highest PASI 75 response rates among nonbiologic systemic therapies across time points. Deucravacitinib PASI 75 response rate (95% CrI) over short-term follow-up was 54.1% (46.5–61.6), within the range of first-generation biologics (etanercept, 39.7% [31.6–48.3]; infliximab, 79.0% [74.0–83.5]). At mid-term follow-up, deucravacitinib PASI 75 increased to 63.3% (58.0–68.4). At long-term follow-up, deucravacitinib PASI 75 was 65.9% (58.0–73.4), comparable to first-generation biologics adalimumab (62.8%; 55.3–69.6) and ustekinumab (68.0%; 64.6–71.5).
Conclusions
Patients receiving deucravacitinib were more likely to achieve PASI 75 response versus apremilast and methotrexate across all time points. The long-term PASI 75 response rate for deucravacitinib was similar to those of adalimumab and ustekinumab. The approval of deucravacitinib offers patients the choice of an oral therapy with long-term efficacy similar to that of some biologics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-01034-7.
Keywords: Biologics, Deucravacitinib, Indirect treatment comparison, Network meta-analysis, Non-biologics, Psoriasis
Key Summary Points
| This network meta-analysis compared 47 phase 3 randomized controlled trials (RCTs) of systemic treatments at short-term (10–16 weeks), mid-term (24–28 weeks), and long-term (44–60 weeks) time points. |
| Deucravacitinib demonstrated superior efficacy in achieving short-, mid-, and long-term Psoriasis Area and Severity Index (PASI 75) response versus methotrexate and apremilast, and long-term versus methotrexate. |
| Short- and long-term response rates for deucravacitinib were within the range of first-generation biologics; long-term response was similar to that of adalimumab and ustekinumab. |
| Deucravacitinib appears to offer long-term efficacy similar to some biologics, with convenient oral administration. |
Introduction
A growing number of systemic therapies are available for the treatment of moderate to severe plaque psoriasis, offering different drug classes, mechanisms of action, routes of administration, and dosing schedules. The biologics include first-generation tumor necrosis factor inhibitors (TNFis; i.e., adalimumab, etanercept, and infliximab) and the interleukin [IL]-12/23 inhibitor ustekinumab, and recently approved second-generation treatments, including the IL-17 inhibitors (i.e., bimekizumab, brodalumab, ixekizumab, and secukinumab) and IL-23 inhibitors (i.e., guselkumab, risankizumab, and tildrakizumab). However, other than the introduction of apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, few advancements have been made in nonbiologics, particularly regarding targeted oral therapies. Deucravacitinib, an oral, selective, first-in-class allosteric tyrosine kinase 2 (TYK2) inhibitor, is approved in the USA, European Union, and other countries for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy.
Deucravacitinib demonstrated superior efficacy compared with apremilast and placebo in two recent phase 3 randomized controlled trials (RCTs), POETYK PSO-1 and POETYK PSO-2 [1, 2]. However, head-to-head trials were not feasible for all possible comparisons, and few studies have directly compared treatments for patients with moderate to severe plaque psoriasis [3–11]. Systematic literature reviews (SLRs) and network meta-analyses (NMAs) are used to evaluate published literature and indirectly compare relative effects of various therapies [12].
The objective of the current analysis was to compare deucravacitinib with other approved systemic treatments for moderate to severe plaque psoriasis using updated evidence.
Methods
Systematic Literature Review
This SLR of RCTs of treatments for plaque psoriasis was conducted according to a prespecified protocol (available through the corresponding author). It was conducted and reported in accordance with the standards set forth in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Table S1 in the supplementary material) [13].
Compliance with Ethics Guidelines
This study was based on previously published studies. All data were obtained from publicly accessible databases (Medline, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and PsycINFO). It contained no newly conducted research involving human participants or animals performed by any of the authors and complied with the Helsinki Declaration of 1964 and all amendments.
Information Sources and Search Strategies
Systematic searches of electronic databases for English-language publications of RCTs involving systemic treatments for moderate to severe plaque psoriasis were performed with a cutoff date of October 2021. A combination of free-text and controlled-vocabulary search terms was used for plaque psoriasis, relevant comparator treatments, and study design filters (Table S2). Additionally, relevant conference proceedings and clinical trial registries supplemented the electronic searches.
Study Selection and Data Extraction
Eligible studies were RCTs (phase 2, 3, 4, or follow-up studies) that included adults (aged ≥ 18 years) with moderate to severe plaque psoriasis. When psoriasis severity was unspecified, it was determined on the basis of reported Physician Global Assessment (PGA), PASI, body surface area (BSA), and Dermatology Life Quality Index (DLQI) criteria (PGA ≥ 3, PASI ≥ 10, BSA ≥ 10, or DLQI ≥ 10). Studies presenting mixed disease severity (i.e., mild, moderate, severe) were considered for inclusion if at least 80% of the population had moderate to severe plaque psoriasis; studies not reporting psoriasis severity were excluded. Studies were included if they reported 50%, 75%, 90%, or 100% improvement in PASI (PASI 50, 75, 90, 100) from baseline in head-to-head comparisons between any systemic therapies and/or placebo. Comparators of interest included approved treatments for plaque psoriasis with eligible dosages consistent with labels approved by the US Food and Drug Administration or European Medicines Agency: TNFis (i.e., adalimumab, etanercept, or infliximab), IL-17 inhibitors (i.e., brodalumab, ixekizumab, secukinumab, or bimekizumab), IL-23 inhibitors (i.e., risankizumab, tildrakizumab, or guselkumab), an IL-12/23 inhibitor (i.e., ustekinumab), and systemic nonbiologics (i.e., deucravacitinib, apremilast, methotrexate, acitretin, or cyclosporine). The inclusion and exclusion criteria are described in Table S3.
Two reviewers screened the titles and abstracts of studies retrieved by the search strategies according to the predefined eligibility criteria. Full-text articles deemed potentially relevant at title and abstract screening were retrieved and assessed for eligibility independently by two reviewers. At all stages of the study selection process, multiple reviewers worked independently as part of the review team; disagreements were resolved by a third, senior researcher (AC, AK, or DJ).
Data elements extracted from the included studies comprised study and patient characteristics (e.g., trial phase, imputation method, sample size, age, sex), treatment regimen, and PASI outcomes assessed at weeks 10–16 (short-term), 24–28 (mid-term), and 44–60 (long-term) time points, where reported. Data on subgroups of interest (i.e., previous use of biologic therapy) were also extracted, if available. The data were extracted by one reviewer into a customized data extraction form, and independently validated by a second, senior investigator (AC, AK, or DJ). Unique studies reported in multiple publications were linked to a primary publication and had data extracted as one study.
Feasibility Assessment
A feasibility assessment was performed to ensure the two main NMA assumptions (consistency and similarity) were met across the included RCTs. Specifically, the characteristics of the trials identified in the SLR and connected in the network (i.e., study design, patient characteristics, populations, interventions and comparators, and outcomes) were assessed to determine whether they were similar enough to be quantitatively synthesized and whether there was any imbalance in potential effect modifiers.
For efficacy analyses beyond the primary trial endpoint (usually, 10–16 weeks for most comparators), additional considerations were required as a result of variation in study designs over the maintenance periods. Therefore, the NMAs of mid-term and long-term PASI outcomes considered only active-controlled trials with treat-through designs in which all patients assigned to active treatment received that treatment through the end of the evaluation period, regardless of response at an earlier time point. The analyses also assumed that placebo response rates at the end of induction could be carried forward through the end of the evaluation period; this assumption allowed for indirect comparisons via placebo and adjusted for baseline risk in the PASI NMAs [14].
Finally, it was assumed that any minor differences in trial design based on phase, blinding, and randomization methods, etc., would not impact the relative treatment effects. No trials were excluded from the analyses as a result of poor study quality.
NMA Methodology
A multinomial (probit) Bayesian NMA of PASI 50, 75, 90, or 100 was conducted to estimate the relative effects of deucravacitinib with other systemic treatments at short-, mid-, and long-term time points. The main analysis included phase 3 RCTs that applied a nonresponder imputation (NRI) method and were either multinational or conducted in a country with diverse ethnicity so that trials had comparable designs, population diversity, and analytic methods. Because exposure to prior treatment could affect response to subsequent therapy, a subgroup analysis of biologic-naive patients was also conducted using a binomial (logit) Bayesian NMA of PASI 75 at the short-term time point. A subgroup analysis for patients previously treated with biologics was planned but not conducted because of a lack of data.
The analyses were conducted using a modified multinomial model that considers variability around the chance that each treatment achieves the next-highest PASI threshold by adding a random-effects component that allows treatments to have different efficacies for different levels of PASI (known as the REZ multinomial model) [15]. Adjustment for baseline risk (i.e., placebo response) accounted for the impact of the variation of absolute placebo responses in the estimates of relative effects across treatments [15–20]. Goodness-of-fit of the different analytic models was compared using the posterior mean residual deviance and deviance information criteria. Results from placebo-adjusted REZ multinomial models underpinned the interpretation of the main analyses as supported by clinical recommendations [18, 21]. The Bayesian NMAs of multinomial models were conducted in JAGS (version 4.3.0), and binomial NMAs were conducted in OpenBUGS (version 3.2.3) with noninformative priors.
All Bayesian analyses were carried out with Markov chain Monte Carlo simulations. Odds ratios (ORs) and the median and 2.5th and 97.5th percentiles of the posterior samples for each effect parameter were used as an estimate of the relative effect and its 95% credible interval (CrI). An estimated probability and corresponding 95% CrI for achieving a PASI response (i.e., PASI 50, 75, 90, or 100) for each treatment were calculated relative to the anchor placebo response rate of PASI 50. To ensure consistency, sensitivity analyses were conducted including all RCTs identified by the SLR and considered eligible for inclusion in the NMA (i.e., without restrictions to trial phase, imputation method, geography, or ethnicity).
For each treatment, the number needed to treat (NNT) to achieve one additional PASI 75 response relative to supportive care was calculated as the reciprocal of the difference in estimated PASI 75 response rates between the biologic treatment and placebo [17].
Risk of Bias
The quality of the included evidence was assessed using the Cochrane Risk of Bias (RoB) 2.0 tool [22]. The risk of bias in each of five domains was classified into three categories (low, some concerns, high), and an overall score was assigned following the algorithm guidance of the RoB 2.0 tool [23].
Results
From 7487 records identified through the searches, 96 unique studies were included in the review, and 47 studies were included in the main analysis (Fig. S1; Table S4) [1–7, 9, 11, 24–104]. Studies of acitretin or cyclosporine were not included in the main analysis because these studies did not meet the inclusion criteria. All studies included in the main analysis reported PASI outcomes at short-term follow-up, 28 reported PASI outcomes at mid-term follow-up, and 20 reported PASI outcomes at long-term follow-up.
Across studies, the average age of patients ranged from 39 to 53 years, and the majority of patients were male. Mean baseline PASI scores ranged from 11 to 29, and exposure to previous biologic therapy varied from 0 to 51%. Trial populations were generally similar with respect to disease severity at baseline. In total, 23 active treatments (nonbiologic systemic therapies and/or biologic therapies) were included in the analyses, although not all treatments could be included for all follow-up periods as a result of lack of data or differences in study design. The majority of trials were placebo-controlled in design (n = 28) as opposed to only having an active control arm (n = 19).
Main Analysis
The network diagrams for all comparators of interest are shown in Figs. 1a, 2a, and 3a. Across all follow-up periods, all treatments were more effective than placebo in achieving all levels of PASI response, and the newer IL-17 and IL-23 inhibitors showed the highest PASI 50, 75, 90, and 100 response rates of all treatments included in the analysis.
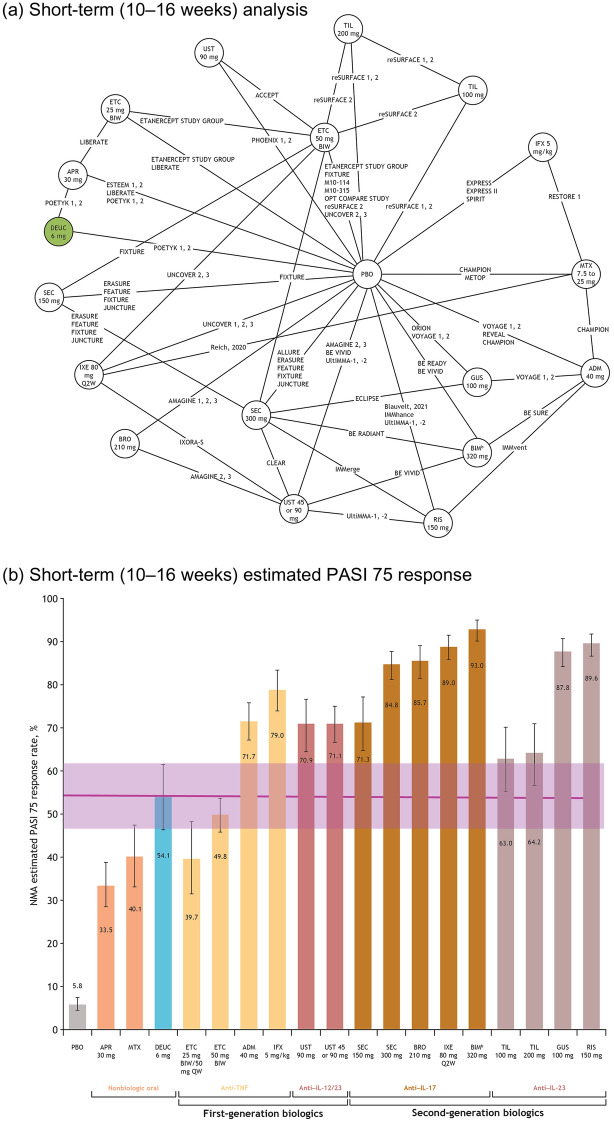
Fig. 1.
Network plot of studies included in the short-term (10–16 weeks) analysis (a), and short-term estimated PASI 75 response,a posterior median, and 95% CrI (b). aAdjusted for placebo response rates. bBIM is not approved for use in the USA. Note: Posterior median value given for each therapy; error bars represent 95% CrI. ADM adalimumab, APR apremilast, BIM bimekizumab, BIW twice weekly, BRO brodalumab, CrI credible interval, DEUC deucravacitinib, ETC etanercept, GUS guselkumab, IFX infliximab, IL interleukin, IXE ixekizumab, MTX methotrexate, NMA network meta-analysis, PASI Psoriasis Area and Severity Index, PBO placebo, QW once every week, Q2W once every 2 weeks, RIS risankizumab, SEC secukinumab, TIL tildrakizumab, TNF tumor necrosis factor, UST ustekinumab
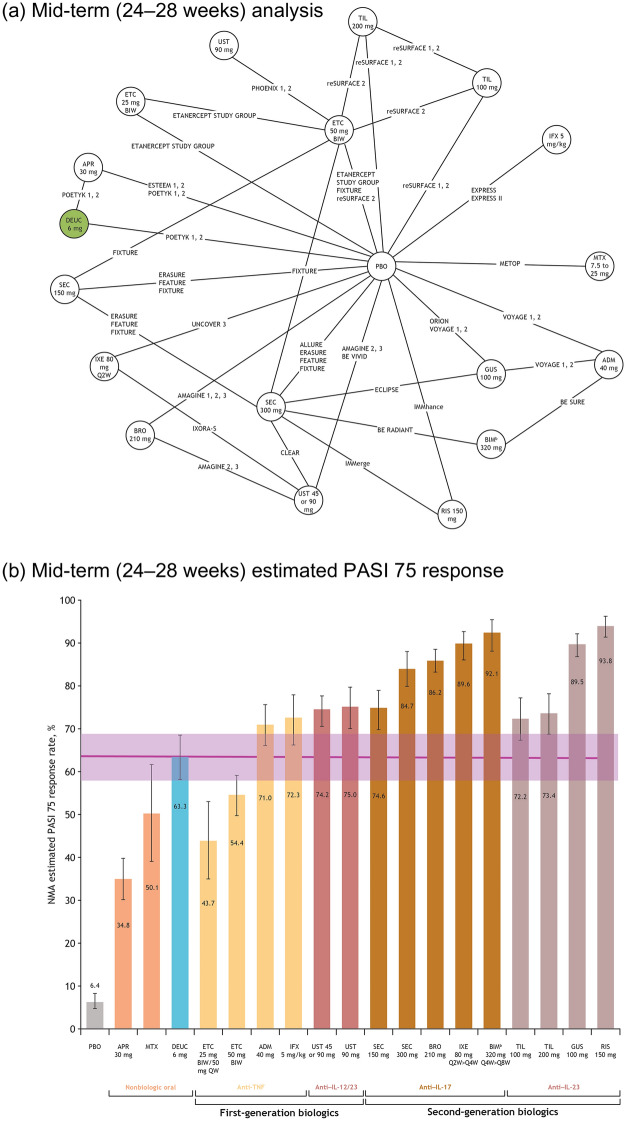
Fig. 2.
Network plot of studies included in the mid-term (24–28 weeks) analysis (a), and mid-term estimated PASI 75 response,a posterior median, and 95% CrI (b). aAdjusted for placebo response rates. bBIM currently is not approved for use in the USA. Note: Posterior median value given for each therapy; error bars represent 95% CrI. ACT acitretin, ADM adalimumab, APR apremilast, BIM bimekizumab, BIW twice weekly, BRO brodalumab, CrI credible interval, DEUC deucravacitinib, ETC etanercept, GUS guselkumab, IFX infliximab, IL interleukin, IXE ixekizumab, MTX methotrexate, NMA network meta-analysis, PASI Psoriasis Area and Severity Index, PBO placebo, QW once every week, Q2W once every 2 weeks, Q4W once every 4 weeks, Q8W once every 8 weeks, RIS risankizumab, SEC secukinumab, TIL tildrakizumab, TNF tumor necrosis factor, UST ustekinumab
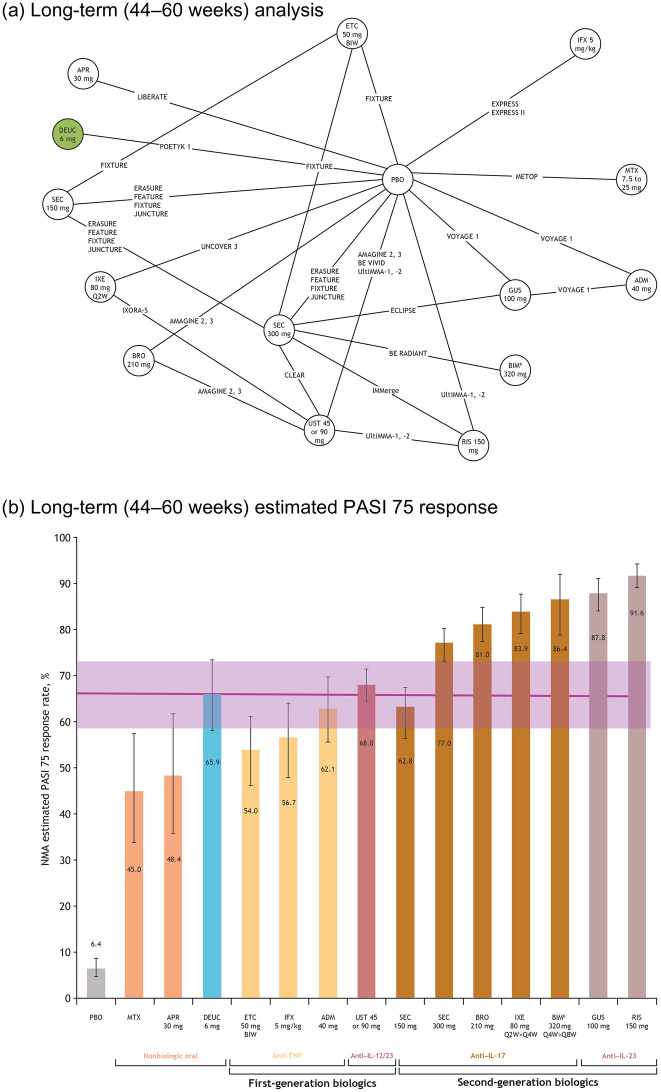
Fig. 3.
Network plot of studies included in the long-term (44–60 weeks) analysis (a), and long-term estimated PASI 75 response,a posterior median, and 95% CrI (b). aAdjusted for placebo response rates.bBIM is not approved for use in the USA. Note: posterior median value given for each therapy; error bars represent 95% CrI. ADM adalimumab, APR apremilast, BIM bimekizumab, BIW twice weekly, BRO brodalumab, CrI credible interval, DEUC deucravacitinib, ETC etanercept, GUS guselkumab, IFX infliximab, IL interleukin, IXE ixekizumab, MTX methotrexate, NMA network meta-analysis, PASI Psoriasis Area and Severity Index, PBO placebo, Q2W every 2 weeks, Q4W once every 4 weeks, Q8W once every 8 weeks, RIS risankizumab, SEC secukinumab, TIL tildrakizumab, TNF tumor necrosis factor, UST ustekinumab
At the short-term follow-up, the estimated PASI 75 response rate (95% CrI) for deucravacitinib (54.1% [46.5–61.6]) was higher than that for etanercept 50 mg (49.8% [45.9–53.8]), methotrexate (40.1% [33.0–47.5]), etanercept 25 mg (39.7% [31.6–48.3]), and apremilast (33.5% [28.6–38.7]) (Fig. 1b). The PASI 75 response of deucravacitinib was in the range of response rates estimated for first-generation biologics (39.7–79.0%). The IL inhibitors secukinumab 300 mg, guselkumab, ixekizumab, brodalumab, risankizumab, and bimekizumab were the most effective treatments, with an estimated PASI 75 response rate of 85% or higher. These trends were similar for PASI 50, 90, and 100 (Table S5).
The PASI 75 response rate (95% CrI) in patients taking deucravacitinib increased in the mid-term analysis to 63.3% (58.0–68.4; Fig. 2b). This response rate (95% CrI) was greater than that for patients taking the nonbiologic treatments apremilast (34.8% [30.1–39.8]) or methotrexate (50.1% [39.0–61.5]), as well as for both doses of the TNFi etanercept (25 mg, 43.7% [35.0–53.0]; 50 mg, 54.4% [49.5–59.0]). These trends were similar for PASI 50, 90, and 100 (Table S6).
In the long-term treatment follow-up, the PASI 75 response rate (95% CrI) in patients taking deucravacitinib was 65.9% (58.0–73.4). This response rate continued to be higher than that in patients receiving the nonbiologic agents, methotrexate (45.0% [33.8–57.3]) and apremilast (48.4% [35.7–61.6]), and the TNFis, etanercept 50 mg (54.0% [46.1–61.1]) and infliximab (56.7% [47.7–63.9]; Fig. 3b). The PASI 75 response rate (95% CrI) for deucravacitinib was comparable to that of some biologics: the IL-17 inhibitor secukinumab (62.8%; [CrI, 55.3–69.6]), the TNFi adalimumab (62.1% [56.3–67.4]), and the IL-12/23 inhibitor ustekinumab (68.0% [64.6–71.5]). These trends were similar for PASI 50, 90, and 100 (Table S7).
At both the short- and mid-term time points, patients receiving deucravacitinib were more likely to achieve a PASI 75 response rate (OR > 1 and P < 0.05) than those receiving placebo, apremilast, methotrexate, or etanercept 25 mg, as well as etanercept 50 mg at the mid-term time point (Fig. 4a, b). At the long-term time point, deucravacitinib was more likely to result in a PASI 75 response than placebo, methotrexate, apremilast, etanercept 50 mg, infliximab, adalimumab, or secukinumab 150 mg (Fig. 4c). These trends generally held for PASI 50, 90, and 100 response rates as short-, mid-, and long-term time points (Figs. S2–S4).
Fig. 4.
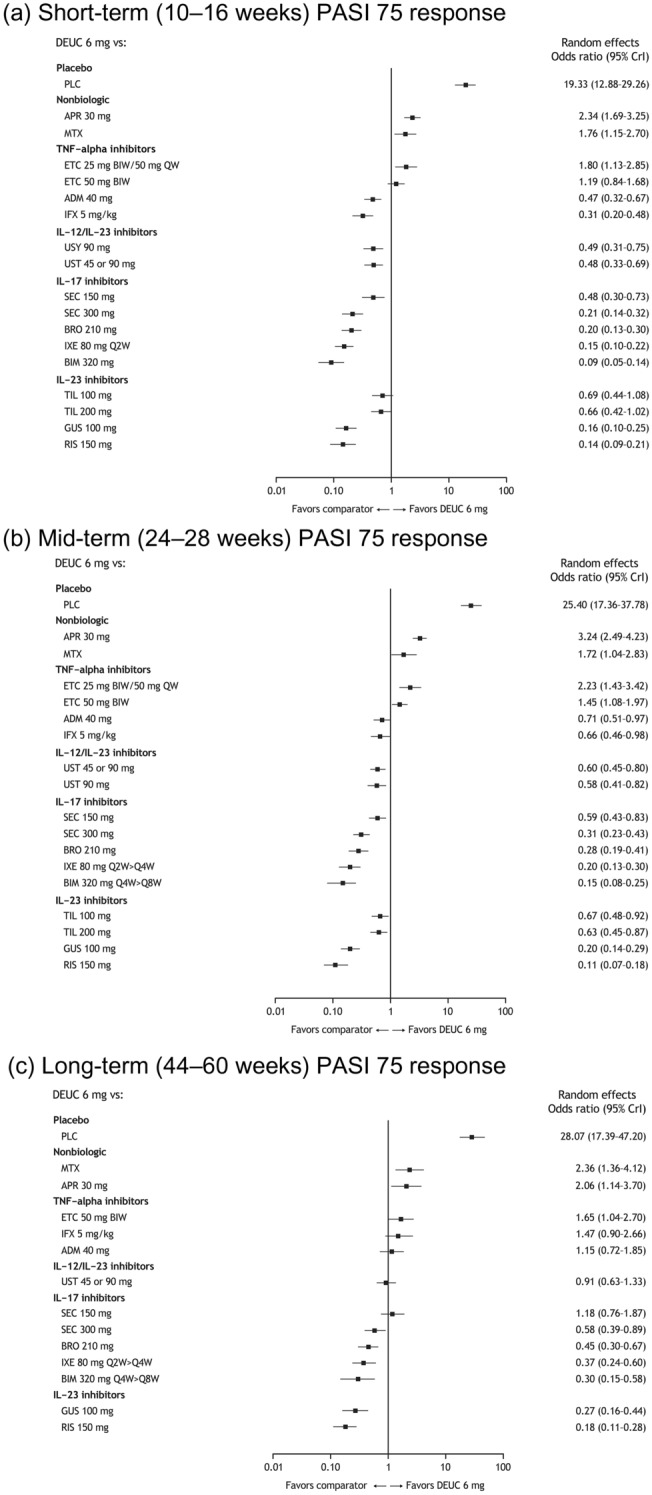
Estimated odds ratios from the network meta-analysis for (a) short-term (10–16 weeks) PASI 75, (b) mid-term (24–28 weeks) PASI 75, and (c) long-term (44–60 weeks) PASI 75. ADM adalimumab, APR apremilast, BIM bimekizumab, BIW twice weekly, BRO brodalumab, CrI credible interval, DEUC deucravacitinib, ETC etanercept, GUS guselkumab, IFX infliximab, IL interleukin, IXE ixekizumab, MTX methotrexate, NMA network meta-analysis, PASI Psoriasis Area and Severity Index, PBO placebo, Q2W every 2 weeks, Q4W once every 4 weeks, Q8W once every 8 weeks, RIS risankizumab, SEC secukinumab, TIL tildrakizumab, TNF tumor necrosis factor, UST ustekinumab
Results of the sensitivity analysis were similar to the findings of the main analysis, thus reinforcing the robustness of the NMA results (Figs. S5–S7).
In the short-term subgroup analysis of biologic-naive patients receiving treatment with deucravacitinib, PASI 75 response rates (95% confidence interval [CI]) for patients receiving treatment with deucravacitinib were higher (54% [42.0–64.0]) than those taking apremilast (38% [28.0–48.0]), methotrexate (30% [19.0–45.0]), etanercept 25 mg (42% [31.0–54.0]), and etanercept 50 mg (54% [39.0–68.0]; Fig. S8).
Compared with placebo, the NNT to achieve PASI 75 at weeks 44 to 60 was 1.68 for deucravacitinib, 1.78 for adalimumab, 1.80 for secukinumab 150 mg, 1.99 for infliximab, 2.11 for etanercept, and 2.38 for apremilast (Tables S8–S10).
Risk of Bias
Of the 47 studies included in the global analysis, 22 (47%) were rated as having an overall low risk of bias, 19 (40%) as having some concerns, and 6 (13%) at high risk for bias (Table S11). The main drivers of risk for bias were missing outcome data (4 studies [9%] at high risk of bias) and deviations from the intended interventions (3 studies [6%] at high risk of bias).
Discussion
This NMA indirectly compared plaque psoriasis treatments by examining relative efficacy response probability rates obtained from published clinical studies. For a comprehensive analysis, results from short-, mid-, and long-term follow-up were assessed. Overall, deucravacitinib demonstrated higher efficacy than the nonbiologics methotrexate and apremilast at all time points. Additionally, the PASI 75 response rate for deucravacitinib was in the range of first-generation biologics at the short-term time point and was higher at the mid-term time point. With continued treatment, the efficacy of deucravacitinib was comparable to that of the most effective first-generation biologics, adalimumab and ustekinumab, at approximately 1 year. The NNT to achieve PASI 75 for deucravacitinib was comparable to that of other biologic and nonbiologic therapies at all time points.
Direct comparisons of clinical efficacy among plaque psoriasis treatments are limited. This NMA builds on previous indirect comparisons [14, 17, 105, 106] by including all approved nonbiologics and first-generation agents through the newest classes of IL-17 and IL-23 inhibitors and the most recently approved TYK2 inhibitor, deucravacitinib. The findings of the current NMA, with the addition of comparisons to deucravacitinib, are generally consistent with the literature, confirming that the newest generation of therapies had the highest efficacy [14, 17]. Notably, the response rates in active-controlled trials (i.e., those with no placebo arm) [4, 11, 24–28] tend to be higher than those reported in placebo-controlled trials for the same treatments. Although both deucravacitinib trials were placebo controlled, our analysis included both placebo-controlled and active-controlled trials to be conservative and comprehensive.
The statistical methods used in this NMA strengthened our analysis, as did the study selection criteria, which ensured comparability of study design, population diversity, and imputation methods among studies. Additionally, the inclusion of analyses for short-, mid-, and long-term follow-up provided a more complete comparison of time to peak efficacy and maintenance of response. NMAs are useful for evaluating numerous clinical studies using different methodologies to compare treatments, although quality criteria, such as a standard treatment duration, study phase, or study methods, may vary [12, 106]. One strength of the current NMA was the attention paid to quality criteria in study selection and in comparisons between study methodologies and results reporting.
Although the most recently approved biologics provide high efficacy rates, a proportion of patients, especially those who are biologic naive or who have needle aversion, may prefer a safe and effective oral treatment option. Route of administration may be a key driver for treatment choice, with patients willing to trade some degree of efficacy for the convenience of oral therapy [107]. Despite unsatisfactory efficacy, earlier nonbiologic therapies are widely used for moderate to severe psoriasis [108]. Results of this analysis indicate that, as an oral treatment with long-term efficacy comparable to that of biologics, deucravacitinib offers a valuable new therapeutic option for patients with moderate to severe plaque psoriasis.
This analysis should be interpreted in light of several limitations. All SLRs are limited by search strategies used, and they all rely on the accuracy of the databases searched and clear descriptions of the trials and populations in titles and abstracts. To mitigate this inherent limitation, reference lists of previously published SLRs on the same population were reviewed to identify any potential references that may have been missed in the database searches. In the mid- and long-term NMAs, the anchor rate for the baseline risk-adjusted analysis was based on a treat-through analysis approach using the short-term placebo data across all time points. This approach ensured the use of similar placebo data across all trials and enabled a connected network that included most active treatments of interest; however, the treat-through scenario was limited only to patients who remained on their initial assigned treatment through 1 year. This may not align with true clinical practice as patients may cycle on or off different regimens over time. In addition, a safety comparison was not conducted for the benefit–risk assessment. Comparing rates of overall adverse events (AEs) would not be meaningful as individual AEs are not of equal importance (e.g., acne vs malignancy), and the NMA was not powered to analyze specific AEs of interest. It should be noted that deucravacitinib was well tolerated with a low rate of discontinuations related to AEs in the pivotal trials [1, 2] and it showed a consistent safety profile in a long-term extension trial [109]. Future long-term studies with large sample sizes are needed to further assess the long-term comparative safety of systemic treatments for plaque psoriasis.
Although the maintenance of response was not compared in this study, it is an important endpoint to consider for treatment choice. In POETYK PSO-1, 82% of patients who achieved PASI 75 and 74% who achieved PASI 90 at week 24 maintained that response at week 52 [110]. This maintenance of response appears to be greater than that reported for TNFis [111–113], and in the range of that reported for IL-17 [114–117] and IL-23 inhibitors [118–120]. Since plaque psoriasis is a chronic condition, future analyses are needed to provide longer-term data.
Conclusions
Among oral nonbiologic treatments, deucravacitinib provided the best efficacy across all time points. The PASI 75 response rates for deucravacitinib were within the range of those of first-generation biologics at short- and mid-term time points, and the long-term PASI 75 response rate was similar to that of adalimumab and ustekinumab. Deucravacitinib appears to offer long-term efficacy similar to that of some biologics with the convenience of an oral therapy. Longer-term data are needed since plaque psoriasis is a chronic condition.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing and Editorial Assistance
Medical writing and editorial assistance were provided by Cheryl Jones of Peloton Advantage, LLC, an OPEN Health company, and funded by Bristol Myers Squibb.
Author Contributions
Yichen Zhong, Joe Zhuo, Allie Cichewicz, Ananth Kadambi, Daniela Junqueira, Renata Kisa, and Matthias Augustin contributed to the literature search and methodology design. Allie Cichewicz, Daniela Junqueira, and Tracy Westley participated in the collection and assembly of data. Allie Cichewicz, Daniela Junqueira, Tracy Westley, and Ananth Kadambi participated in data analysis. April Armstrong, Richard Warren, Yichen Zhong, Joe Zhuo, Allie Cichewicz, Ananth Kadambi, Daniela Junqueira, Tracy Westley, Renata Kisa, Carolin Daamen and Matthias Augustin contributed to data interpretation. All authors collaborated in the preparation of the manuscript, supported by a professional medical writer funded by Bristol Myers Squibb, and critically reviewed and provided revisions to the manuscript. All authors had access to the literature search results and articles. All authors granted final approval of the manuscript for submission.
Funding
This work was sponsored by Bristol Myers Squibb. This study sponsor also funded the journal’s Rapid Service Fee.
Data Availability
All data generated or analyzed during this study are included in this published article and supplementary information files. The data were obtained through searches of publicly accessible electronic databases (Medline, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and PsycINFO). Inclusion criteria and search strings are specified in the Methods section, and Supplementary Materials would allow other researchers to identify the same studies used in this analysis.
Declarations
Conflict of Interest
April W. Armstrong has received grants and personal fees from AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Leo Pharma, and Novartis; personal fees from Boehringer Ingelheim/Parexel, Celgene, Dermavant, Genentech, GlaxoSmithKline, Menlo Therapeutics, Merck, Modernizing Medicine, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Science 37, Sun Pharma, and Valeant, and grants from Dermira, Kyowa Hakko Kirin, and UCB. Richard Warren has received research grants from AbbVie, Almirall, Amgen, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, and UCB; and consulting fees from AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, DiCE, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, UCB, and Union. Yichen Zhong, Joe Zhuo, Renata Kisa, and Carolin Daamen are employees of and shareholders in Bristol Myers Squibb. Allie Cichewicz, Ananth Kadambi, Daniela Junqueira, and Tracy Westley are employed by Evidera, a part of Thermo Fisher Scientific, a company that provides consulting and other research services to Bristol Myers Squibb. Matthias Augustin has served on advisory boards for AbbVie, Amgen, Boehringer Ingelheim, Janssen Biotech, and Leo Pharma; has received consulting fees from AbbVie, Amgen, Eli Lilly, Janssen Biotech, Leo Pharma, Novartis, Sun Pharma, and UCB; has received honoraria from AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Janssen Biotech, Leo Pharma, Novartis, Sun Pharma, and UCB; has served as an investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen Biotech, Leo Pharma, Merck, Novartis, Sun Pharma, and UCB; has received research grants from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen Biotech, Leo Pharma, Merck, and Sun Pharma; and has served as a speaker for AbbVie, Amgen, Janssen Biotech, Leo Pharma, Sun Pharma, and UCB.
Ethical Approval
This study was based on previously published studies. All data were obtained from publicly accessible databases (Medline, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and PsycINFO). It contained no newly conducted research involving human participants or animals performed by any of the authors. This study was conducted in compliance with the Helsinki Declaration of 1964 and all amendments.
References
- 1.Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29–39. doi: 10.1016/j.jaad.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, Program fOr Evaluation of TYK2 inhibitor psoriasis second phase 3 trial. J Am Acad Dermatol. 2023;88(1):40–51. doi: 10.1016/j.jaad.2022.08.061. [DOI] [PubMed] [Google Scholar]
- 3.Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661. doi: 10.1016/S0140-6736(18)31713-6. [DOI] [PubMed] [Google Scholar]
- 4.Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–152. doi: 10.1056/NEJMoa2102383. [DOI] [PubMed] [Google Scholar]
- 5.Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–586. doi: 10.1016/S0140-6736(19)30952-3. [DOI] [PubMed] [Google Scholar]
- 6.Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE) J Eur Acad Dermatol Venereol. 2017;31(3):507–517. doi: 10.1111/jdv.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288. doi: 10.1016/S0140-6736(17)31279-5. [DOI] [PubMed] [Google Scholar]
- 8.Blauvelt A, Papp K, Gottlieb A, et al. A head-to-head comparison of ixekizumab vs guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2020;182(6):1348–1358. doi: 10.1111/bjd.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 10.Reich K, Leonardi C, Langley RG, et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: a presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol. 2017;76(3):441–8.e2. doi: 10.1016/j.jaad.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014–1023. doi: 10.1111/bjd.15666. [DOI] [PubMed] [Google Scholar]
- 12.Guelimi R, Metelli S, Sbidian E, et al. Network meta-analysis: methodological points for readers, authors and reviewers. Br J Dermatol. 2022;186(6):917–918. doi: 10.1111/bjd.21013. [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer LM, Cornic L, Levin L, Gibbons C, Møller AH, Jemec GB. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. J Eur Acad Dermatol Venereol. 2019;33(2):355–366. doi: 10.1111/jdv.15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahrbach K, Sarri G, Phillippo DM, et al. Short-term efficacy of biologic therapies in moderate-to-severe plaque psoriasis: a systematic literature review and an enhanced multinomial network meta-analysis. Dermatol Ther (Heidelb) 2021;11(6):1965–1998. doi: 10.1007/s13555-021-00602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. National Institute for Health and Care Excellence, 2016. https://www.ncbi.nlm.nih.gov/books/NBK310366/. Accessed 02 May 2023. [PubMed]
- 17.Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269. doi: 10.1001/jamadermatol.2019.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bimekizumab for treating moderate to severe plaque psoriasis [ID2692]. Appraisal Committee Meeting – 7 July 2021. National Institute for Health and Care Excellence, 2021. https://www.nice.org.uk/guidance/ta723/history. Accessed 09 March 2023.
- 19.Wade R, Grosso A, South E, et al. Brodalumab for the treatment of moderate-to-severe plaque psoriasis: an evidence review group evaluation of a NICE single technology appraisal. Pharmacoeconomics. 2019;37(2):131–139. doi: 10.1007/s40273-018-0698-2. [DOI] [PubMed] [Google Scholar]
- 20.Cameron C, Hutton B, Druchok C, et al. Importance of assessing and adjusting for cross-study heterogeneity in network meta-analysis: a case study of psoriasis. J Comp Eff Res. 2018;7(11):1037–1051. doi: 10.2217/cer-2018-0065. [DOI] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence . Brodalumab for treating moderate to severe plaque psoriasis: technology appraisal guidance [TA511] National Institute for Health and Care Excellence; 2018. [Google Scholar]
- 22.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Savović J, Page MJ, Sterne JAC. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). Risk-of-Bias, 2019. https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2. Accessed 13 Jan 2023.
- 24.Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130–141. doi: 10.1056/NEJMoa2102388. [DOI] [PubMed] [Google Scholar]
- 25.Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 27.Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839. doi: 10.1016/S0140-6736(19)31773-8. [DOI] [PubMed] [Google Scholar]
- 28.Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50–59. doi: 10.1111/bjd.19341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 30.Sigurgeirsson B, Schäkel K, Hong CH, et al. Efficacy, tolerability, patient usability, and satisfaction with a 2 mL pre-filled syringe containing secukinumab 300 mg in patients with moderate to severe plaque psoriasis: results from the phase 3 randomized, double-blind, placebo-controlled ALLURE study. J Dermatol Treat. 2022;33(3):1718–1726. doi: 10.1080/09546634.2021.1902925. [DOI] [PubMed] [Google Scholar]
- 31.AlMutairi N, Eassa B. Comparing the efficacy and safety of IL-17 inhibitors for treatment of moderate-to-severe psoriasis: a randomized double blind pilot study with a review of literature. Postepy Dermatol Alergol. 2021;38(2):281–288. doi: 10.5114/ada.2019.91496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. doi: 10.1111/bjd.14493. [DOI] [PubMed] [Google Scholar]
- 33.Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a phase II/III randomized controlled study. J Dermatol. 2010;37(4):299–310. doi: 10.1111/j.1346-8138.2009.00748.x. [DOI] [PubMed] [Google Scholar]
- 34.Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79(2):277–86.e10. doi: 10.1016/j.jaad.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 35.Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–486. doi: 10.1016/S0140-6736(21)00126-4. [DOI] [PubMed] [Google Scholar]
- 36.Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–498. doi: 10.1016/S0140-6736(21)00125-2. [DOI] [PubMed] [Google Scholar]
- 37.Blauvelt A, Gordon KB, Lee P, et al. Efficacy, safety, usability, and acceptability of risankizumab 150 mg formulation administered by prefilled syringe or by an autoinjector for moderate to severe plaque psoriasis. J Dermatol Treat. 2022;33(4):2085–2093. doi: 10.1080/09546634.2021.1914812. [DOI] [PubMed] [Google Scholar]
- 38.Mrowietz U, Szepietowski JC, Loewe R, et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm(®) - and placebo-controlled trial (BRIDGE) Br J Dermatol. 2017;176(3):615–623. doi: 10.1111/bjd.14947. [DOI] [PubMed] [Google Scholar]
- 39.Cai L, Gu J, Zheng J, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. 2017;31(1):89–95. doi: 10.1111/jdv.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krueger JG, Wharton KA, Jr, Schlitt T, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144(3):750–763. doi: 10.1016/j.jaci.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 41.Cai L, Zhang JZ, Yao X, et al. Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in Chinese patients with moderate to severe plaque psoriasis. Chin Med J (Engl) 2020;133(22):2665–2673. doi: 10.1097/CM9.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caproni M, Antiga E, Melani L, Volpi W, Del Bianco E, Fabbri P. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. J Clin Immunol. 2009;29(2):210–214. doi: 10.1007/s10875-008-9233-0. [DOI] [PubMed] [Google Scholar]
- 43.von Stebut E, Reich K, Thaçi D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139(5):1054–1062. doi: 10.1016/j.jid.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 44.Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158(3):558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 45.Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357(9271):1842–1847. doi: 10.1016/S0140-6736(00)04954-0. [DOI] [PubMed] [Google Scholar]
- 46.Lebwohl M, Blauvelt A, Paul C, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT) J Am Acad Dermatol. 2018;79(2):266–76.e5. doi: 10.1016/j.jaad.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Gottlieb AB, Blauvelt A, Thaçi D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2) J Am Acad Dermatol. 2018;79(2):302–14.e6. doi: 10.1016/j.jaad.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Bagel J, Nia J, Hashim PW, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-week CLARITY results) Dermatol Ther (Heidelb) 2018;8(4):571–579. doi: 10.1007/s13555-018-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 50.Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 51.Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152(6):1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- 52.Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154(6):1161–1168. doi: 10.1111/j.1365-2133.2006.07237.x. [DOI] [PubMed] [Google Scholar]
- 53.Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31.e1–15. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 54.Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE) Br J Dermatol. 2015;172(2):484–493. doi: 10.1111/bjd.13348. [DOI] [PubMed] [Google Scholar]
- 55.Gottlieb AB, Blauvelt A, Prinz JC, et al. Secukinumab self-administration by prefilled syringe maintains reduction of plaque psoriasis severity over 52 weeks: results of the FEATURE trial. J Drugs Dermatol. 2016;15(10):1226–1234. [PubMed] [Google Scholar]
- 56.Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol. 2008;158(1):116–121. doi: 10.1111/j.1365-2133.2007.08284.x. [DOI] [PubMed] [Google Scholar]
- 57.Gisondi P, Del Giglio M, Cotena C, Girolomoni G. Combining etanercept and acitretin in the therapy of chronic plaque psoriasis: a 24-week, randomized, controlled, investigator-blinded pilot trial. Br J Dermatol. 2008;158(6):1345–1349. doi: 10.1111/j.1365-2133.2008.08564.x. [DOI] [PubMed] [Google Scholar]
- 58.Goldminz AM, Suarez-Farinas M, Wang AC, Dumont N, Krueger JG, Gottlieb AB. CCL20 and IL22 messenger RNA expression after adalimumab vs methotrexate treatment of psoriasis: a randomized clinical trial. JAMA Dermatol. 2015;151(8):837–846. doi: 10.1001/jamadermatol.2015.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139(12):1627–1632. doi: 10.1001/archderm.139.12.1627. [DOI] [PubMed] [Google Scholar]
- 60.Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349(7):658–665. doi: 10.1056/NEJMoa021359. [DOI] [PubMed] [Google Scholar]
- 61.Igarashi A, Kato T, Kato M, Song M, Nakagawa H. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39(3):242–252. doi: 10.1111/j.1346-8138.2011.01347.x. [DOI] [PubMed] [Google Scholar]
- 62.Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):649–658. doi: 10.1001/jamadermatol.2020.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE) J Eur Acad Dermatol Venereol. 2015;29(6):1082–1090. doi: 10.1111/jdv.12751. [DOI] [PubMed] [Google Scholar]
- 64.Lee JH, Youn JI, Kim TY, et al. A multicenter, randomized, open-label pilot trial assessing the efficacy and safety of etanercept 50 mg twice weekly followed by etanercept 25 mg twice weekly, the combination of etanercept 25 mg twice weekly and acitretin, and acitretin alone in patients with moderate to severe psoriasis. BMC Dermatol. 2016;16(1):11. doi: 10.1186/s12895-016-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349(21):2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 66.Gordon KB, Langley RG, Leonardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55(4):598–606. doi: 10.1016/j.jaad.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 67.Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165(3):652–660. doi: 10.1111/j.1365-2133.2011.10418.x. [DOI] [PubMed] [Google Scholar]
- 68.Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165(3):661–668. doi: 10.1111/j.1365-2133.2011.10419.x. [DOI] [PubMed] [Google Scholar]
- 69.Meffert H, Bräutigam M, Färber L, Weidinger G. Low-dose (1.25 mg/kg) cyclosporin A: treatment of psoriasis and investigation of the influence on lipid profile. Acta Derm Venereol. 1997;77(2):137–141. doi: 10.2340/0001555577137141. [DOI] [PubMed] [Google Scholar]
- 70.Nakagawa H, Niiro H, Ootaki K. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81(1):44–52. doi: 10.1016/j.jdermsci.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 71.Noor SM, Ayub N, Paracha MM. Efficacy and safety of methotrexate versus acitretin in chronic plaque psoriasis. J Postgrad Med Inst. 2017;31(1):4–7. [Google Scholar]
- 72.Ohtsuki M, Okubo Y, Komine M, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in the treatment of Japanese patients with moderate to severe plaque psoriasis: efficacy, safety and tolerability results from a phase 2b randomized controlled trial. J Dermatol. 2017;44(8):873–884. doi: 10.1111/1346-8138.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45(9):1053–1062. doi: 10.1111/1346-8138.14504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–561. doi: 10.1016/S0140-6736(14)62113-9. [DOI] [PubMed] [Google Scholar]
- 75.Ferris LK, Ott E, Jiang J, et al. Efficacy and safety of guselkumab, administered with a novel patient-controlled injector (One-Press), for moderate-to-severe psoriasis: results from the phase 3 ORION study. J Dermatol Treat. 2020;31(2):152–159. doi: 10.1080/09546634.2019.1587145. [DOI] [PubMed] [Google Scholar]
- 76.Papp K, Thaçi D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173(4):930–939. doi: 10.1111/bjd.13932. [DOI] [PubMed] [Google Scholar]
- 77.Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 78.Papp K, Cather JC, Rosoph L, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380(9843):738–746. doi: 10.1016/S0140-6736(12)60642-4. [DOI] [PubMed] [Google Scholar]
- 79.Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 80.Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371(9625):1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 81.de Vries AC, Thio HB, de Kort WJ, et al. A prospective randomized controlled trial comparing infliximab and etanercept in patients with moderate-to-severe chronic plaque-type psoriasis: the Psoriasis Infliximab vs. Etanercept Comparison Evaluation (PIECE) study. Br J Dermatol. 2017;176(3):624–633. doi: 10.1111/bjd.14867. [DOI] [PubMed] [Google Scholar]
- 82.Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab' certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167(1):180–190. doi: 10.1111/j.1365-2133.2012.10941.x. [DOI] [PubMed] [Google Scholar]
- 83.Reich K, Augustin M, Thaçi D, et al. A 24-week multicentre, randomized, open-label, parallel-group study comparing the efficacy and safety of ixekizumab vs. fumaric acid esters and methotrexate in patients with moderate-to-severe plaque psoriasis naive to systemic treatment. Br J Dermatol. 2020;182(4):869–879. doi: 10.1111/bjd.18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barker J, Hoffmann M, Wozel G, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1) Br J Dermatol. 2011;165(5):1109–1117. doi: 10.1111/j.1365-2133.2011.10615.x. [DOI] [PubMed] [Google Scholar]
- 85.Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 86.Rui W, Li X, Zheng J, et al. Efficacy and safety of ixekizumab in Chinese patients with moderate-to-severe plaque psoriasis: 12-week results from a phase 3 study [abstract 25258] J Am Acad Dermatol. 2021;85(3):AB55. doi: 10.1016/j.jaad.2021.06.246. [DOI] [Google Scholar]
- 87.Seo SJ, Shin BS, Lee JH, Jeong H. Efficacy and safety of brodalumab in the Korean population for the treatment of moderate to severe plaque psoriasis: a randomized, phase III, double-blind, placebo-controlled study. J Dermatol. 2021;48(6):807–817. doi: 10.1111/1346-8138.15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gottlieb AB, Evans R, Li S, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51(4):534–542. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 89.Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46(8):686–694. doi: 10.1111/1346-8138.14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 91.Umezawa Y, Sakurai S, Hoshii N, Nakagawa H. Certolizumab pegol for the treatment of moderate to severe plaque psoriasis: 16-week results from a phase 2/3 Japanese study. Dermatol Ther (Heidelb) 2021;11(2):513–528. doi: 10.1007/s13555-021-00494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 93.Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. doi: 10.1016/S0140-6736(15)60125-8. [DOI] [PubMed] [Google Scholar]
- 94.Stein Gold L, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. J Drugs Dermatol. 2018;17(2):221–228. [PubMed] [Google Scholar]
- 95.Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis with lower BSA: week 16 results from the UNVEIL study. J Drugs Dermatol. 2017;16(8):801–808. [PubMed] [Google Scholar]
- 96.van de Kerkhof PC, Segaert S, Lahfa M, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008;159(5):1177–1185. doi: 10.1111/j.1365-2133.2008.08771.x. [DOI] [PubMed] [Google Scholar]
- 97.Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11(6):e007394. doi: 10.1161/CIRCIMAGING.117.007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gelfand JM, Shin DB, Duffin KC, et al. A randomized placebo-controlled trial of secukinumab on aortic vascular inflammation in moderate-to-severe plaque psoriasis (VIP-S) J Invest Dermatol. 2020;140(9):1784–93.e2. doi: 10.1016/j.jid.2020.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gelfand JM, Shin DB, Alavi A, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U trial) J Invest Dermatol. 2020;140(1):85–93.e2. doi: 10.1016/j.jid.2019.07.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi: 10.1016/j.jaad.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 101.Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431. doi: 10.1016/j.jaad.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 102.Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373(2):136–144. doi: 10.1056/NEJMoa1501646. [DOI] [PubMed] [Google Scholar]
- 103.Yang HZ, Wang K, Jin HZ, et al. Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chin Med J (Engl) 2012;125(11):1845–1851. [PubMed] [Google Scholar]
- 104.Warren RB, Mrowietz U, von Kiedrowski R, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10068):528–537. doi: 10.1016/S0140-6736(16)32127-4. [DOI] [PubMed] [Google Scholar]
- 105.Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Augustin M, Valencia López M, Reich K. Network meta-analyses in psoriasis: overview and critical discussion. J Eur Acad Dermatol Venereol. 2021;35(12):2367–2376. doi: 10.1111/jdv.17650. [DOI] [PubMed] [Google Scholar]
- 107.Feldman SR, Moeller AH, Idemyr STE, Gonzalez M. Relative importance of mode of administration in treatment preferences among plaque psoriasis patients in the United States. J Health Econ Outcomes Res. 2016;4(2):141–157. doi: 10.36469/9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lebwohl M, Langley RG, Paul C, et al. Evolution of patient perceptions of psoriatic disease: results from the understanding psoriatic disease leveraging insights for treatment (UPLIFT) survey. Dermatol Ther (Heidelb) 2022;12(1):61–78. doi: 10.1007/s13555-021-00635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Warren BF, Sofen H, Imafuku S, Szepietowski JC, Blauvelt A, Spelman L, et al. Deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [poster P465]. In: Presented at: Annual Congress of the European Academy of Dermatology and Venereology; 12–14 May 2022; Ljubljana, Slovenia.
- 110.Sotyktu [package insert]. Princeton, NJ, USA: Bristol-Myers Squibb Company, 2022.
- 111.Humira [package insert]. North Chicago, IL: Abbott Laboratories (AbbVie), 2021.
- 112.Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc., 2021.
- 113.Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation, 2021.
- 114.Siliq [package insert]. Bridgewater, NJ: Bausch Health US LLC, 2020.
- 115.Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company, 2021.
- 116.Cosentyx [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2021.
- 117.Bimzelx [summary of product characteristics]. Braine-l’Alleud, Belgium: UCB Pharma S.A., 2022.
- 118.Tremfya [package insert]. Horsham, PA: Janssen Biotech, Inc., 2020.
- 119.Skyrizi [package insert]. North Chicago, IL, USA: AbbVie Inc., 2023.
- 120.Ilumya [package insert]. Cranbury, NJ: Sun Pharmaceutical Industries, Inc., 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and supplementary information files. The data were obtained through searches of publicly accessible electronic databases (Medline, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and PsycINFO). Inclusion criteria and search strings are specified in the Methods section, and Supplementary Materials would allow other researchers to identify the same studies used in this analysis.