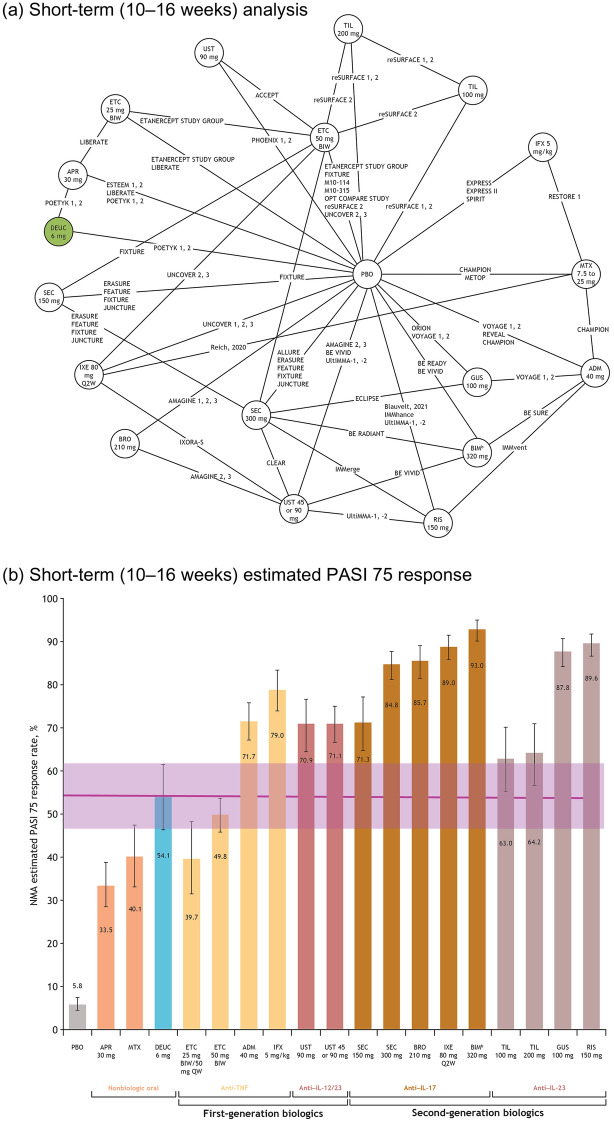
Fig. 1.
Network plot of studies included in the short-term (10–16 weeks) analysis (a), and short-term estimated PASI 75 response,a posterior median, and 95% CrI (b). aAdjusted for placebo response rates. bBIM is not approved for use in the USA. Note: Posterior median value given for each therapy; error bars represent 95% CrI. ADM adalimumab, APR apremilast, BIM bimekizumab, BIW twice weekly, BRO brodalumab, CrI credible interval, DEUC deucravacitinib, ETC etanercept, GUS guselkumab, IFX infliximab, IL interleukin, IXE ixekizumab, MTX methotrexate, NMA network meta-analysis, PASI Psoriasis Area and Severity Index, PBO placebo, QW once every week, Q2W once every 2 weeks, RIS risankizumab, SEC secukinumab, TIL tildrakizumab, TNF tumor necrosis factor, UST ustekinumab