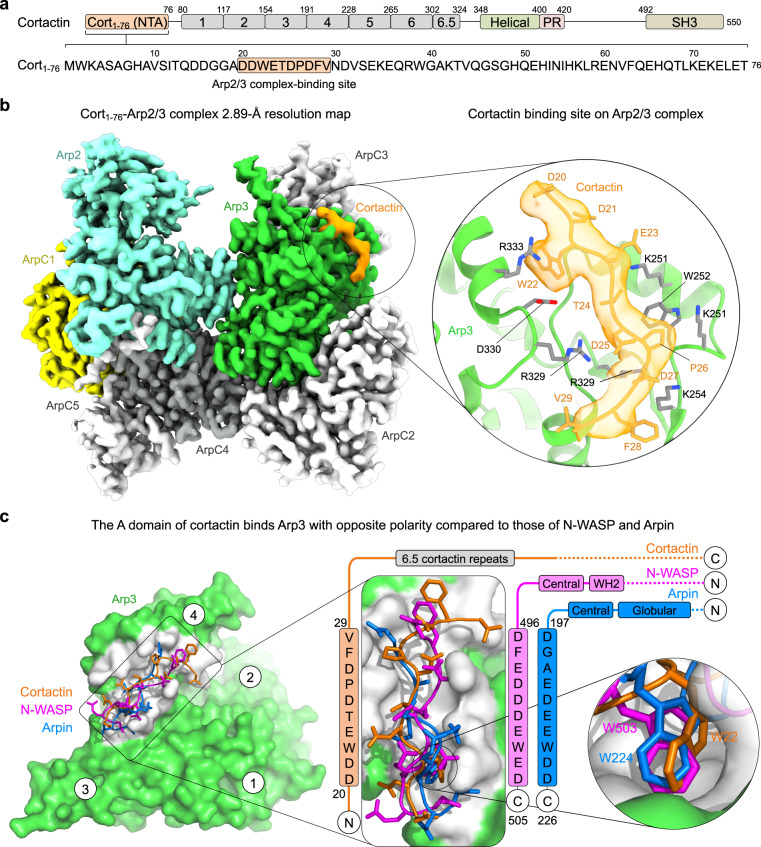
Fig. 1. Cryo-EM structure of the inverted acidic domain of cortactin bound to Arp2/3 complex.
a Domain diagram of human cortactin (UniProt: Q60598), highlighting the sequence of the fragment Cort1-76 used in the cryo-EM structure and residues D20-V29 implicated in Arp2/3 complex binding. b Cryo-EM map of Cort1-76 (orange) bound to Arp2/3 complex. Arp2/3 complex subunits are colored as follows: Arp2, cyan; Arp3, green; ArpC1, yellow; ArpC2, ArpC3, and ArpC5, light gray; ArpC4, dark gray. The inset shows cortactin residues D20-V29 and the corresponding cryo-EM map in orange, and the binding site on Arp3 (green cartoon representation with gray side chains). See also Supplementary Fig. 2a. c Superimposition of the A domains of cortactin (orange), N-WASP (magenta, PDB code 6UHC), and Arpin (blue, PDB code 7JPN) on Arp3 (green and gray). Insets show the binding site (gray) of the A domains and the binding pocket of the conserved tryptophan of the three proteins. The sequences of the three A domains are shown on the side, vertically aligned according to the structures. Note that cortactin binds the site on Arp3 with inverted polarity compared to N-WASP and Arpin.