Abstract
This review explores the potential benefits of taurine in ameliorating the metabolic disorders of obesity and type 2 diabetes (T2D), highlighting the factors that bridge these associations. Relevant articles and studies were reviewed to conduct a comprehensive analysis of the relationship between obesity and the development of T2D and the effect of taurine on those conditions. The loss of normal β-cell function and development of T2D are associated with obesity-derived insulin resistance. The occurrence of diabetes has been linked to the low bioavailability of taurine, which plays critical roles in normal β-cell function, anti-oxidation, and anti-inflammation. The relationships among obesity, insulin resistance, β-cell dysfunction, and T2D are complex and intertwined. Taurine may play a role in ameliorating these metabolic disorders through different pathways, but further research is needed to fully understand its effects and potential as a therapeutic intervention.
Keywords: Diabetes complications, Taurine, Obesity, Inflammation, Beta-cell dysfunction
INTRODUCTION
The global rise in obesity rates is a concerning contemporary issue. In the United States, only approximately one-third of adults have a normal weight, and this trend has also been observed worldwide [1]. Obesity is closely linked to long-term health problems, particularly type 2 diabetes (T2D), which currently affects around 171 million people worldwide, with projections suggesting an increase to 366 million by 2030 [2]. While obesity and T2D are commonly associated with insulin resistance, some individuals with insulin resistance do not experience high blood sugar levels [3]. This is because the pancreas compensates for the reduced insulin efficiency caused by insulin resistance, thereby maintaining normal glucose levels. However, when β-cells fail to produce sufficient insulin, obesity-related insulin resistance can lead to the development of T2D, even in individuals with normal glucose levels. Free circulating non-esterified fatty acids (NEFAs) play a significant role in β-cell dysfunction and insulin resistance [4].
Taurine is a semi-essential amino acid with sulfur content. It constitutes about 0.1% of human body weight and plays vital roles in anti-oxidation, osmoregulation, calcium ion regulation, cell membrane stabilization, and inflammation [5-7]. Dietary intake is necessary to maintain taurine levels since its natural production in the body is relatively low. Insufficient taurine levels have been linked to diabetes, as studies have reported low plasma taurine concentrations in individuals with diabetes [8,9]. In patients with diabetes, Imae et al. [10] observed increased renal excretion of dietary taurine compared to intestinal absorption, leading to reduced taurine levels in the liver. This can be attributed to decreased activity of taurine transporters due to high glucose concentrations. Hansen [11] suggested that elevated sorbitol levels within cells can contribute to taurine reduction. These findings indicate that taurine deficiency may contribute to diabetes in individuals with limited taurine bioavailability. Preclinical studies have shown that taurine supplementation can improve glucose tolerance, insulin secretion, and sensitivity in animal models of T2D. Clinical studies, however, have yielded conflicting results, with some showing no significant effects of taurine supplementation on metabolic syndrome or T2D. Despite these discrepancies, the potential benefits of taurine on various bodily systems, as highlighted in this review, suggest that it may be a promising candidate for diabetes management [12,13].
This narrative review examines the relationships among obesity-induced insulin resistance, β-cell dysfunction, the development of T2D, and the potential therapeutic effects of taurine in addressing these metabolic disorders. We explore the underlying mechanisms of taurine’s action and highlight its bridging role in balancing obesity and diabetic complications. This study is the first to comprehensively explore these connections, adding to the existing literature and providing new insights into the therapeutic potential of taurine.
INSULIN RESISTANCE AND OBESITY
Obesity is one of the most critical factors in the development of different metabolic diseases. Adipose tissue not only stores lipids but also functions as an endocrine gland, thereby modulating metabolism through the release of hormones such as leptin, adiponectin, and pro-inflammatory cytokines, as well as glycerol and NEFAs [14,15]. Conversely, adiponectin enhances insulin sensitivity and fatty acid oxidation through the peroxisome proliferator-activated receptor-α (PPAR-α) and AMP-activated protein kinase (AMPK) pathways [16,17].
Moreover, the enhanced secretion of tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein 1 (MCP-1), and interleukin-6 (IL-6), along with macrophages and other cells in the adipose tissue, leads to an inflamed state that promotes insulin resistance [18]. Cytokines induce insulin resistance, which can be mediated by the suppressor of cytokine signaling proteins and inducible nitric oxide synthase pathways [19]. The secretion of pro-inflammatory cytokines (MCP-1, TNF-α, and IL-6) leads to the recruitment of macrophages and other immune cells, thereby exacerbating inflammation.
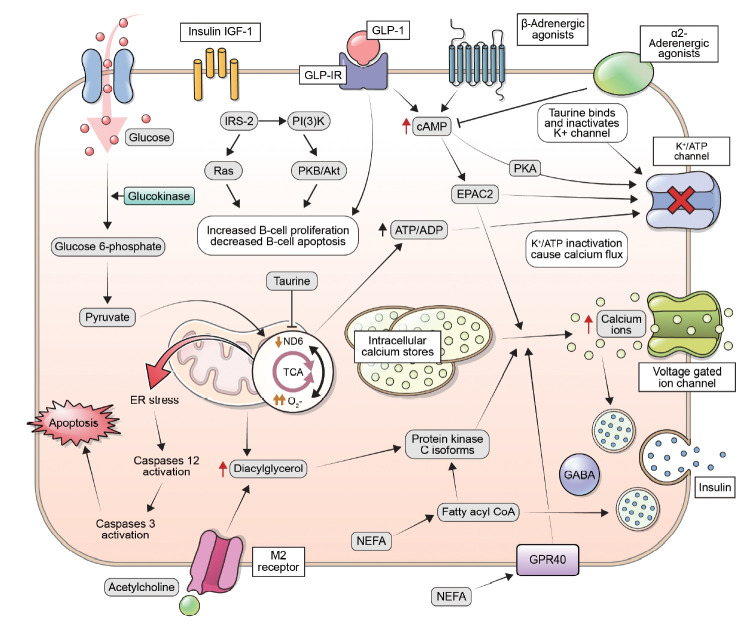
The abundance of NEFAs is a critical factor in regulating insulin sensitivity. Elevated levels of NEFAs are associated with insulin resistance in conditions such as obesity and T2D [20,21]. Even a slight increase in plasma NEFA levels can cause insulin resistance within hours. Conversely, the use of antilipolytic agents (e.g., acipimox), which decrease plasma NEFA levels, can lead to improved insulin-mediated glucose uptake and tolerance. An increase in intracellular NEFA delivery causes NEFAs to compete with glucose for substrate oxidation. This competition inhibits the normal functioning of pyruvate dehydrogenase, phosphofructokinase, and hexokinase II. NEFAs, along with increased intracellular fatty acid metabolites such as fatty acyl-coenzyme A (CoA), contribute to this process. These phosphorylated products have a reduced ability to activate phosphoinositide 3-kinase, thereby inhibiting the downstream signaling pathway of insulin receptors, as elaborated in Fig. 1.
Fig. 1.
Insulin secretion in response to an increase in glucose levels occurs through a process known as glucose-stimulated insulin secretion. This process is mediated by changes in the ratio of adenosine triphosphate (ATP) to adenosine diphosphate (ADP) within the β-cells, which leads to the closure of potassium-ATP channels, depolarization of the plasma membrane, and an increase in cytoplasmic calcium concentrations. These changes ultimately result in the exocytosis of insulin-containing secretory granules. Additionally, in situations where insulin demand is high, β-cells can increase their glucose metabolism through the activation of the enzyme glucokinase. Insulin release is regulated by a variety of mechanisms, including glucose levels, fatty acids, incretins, and nerve signaling. Increased levels of glucose can cause an increase in citrate levels, leading to elevated levels of malonyl-coenzyme A (CoA) and a decrease in carnitine palmitoyl transferase-1 (CPT1) activity. This results in an accumulation of long-chain acyl-CoA and activates protein kinase C (PKC) signaling. Fatty acids can also regulate insulin secretion by signaling through G-protein-coupled receptor 40 (GPR40), or by being metabolized into fatty acyl-CoA and triggering insulin granule exocytosis. The hormone glucagon-like peptide-1 (GLP-1) enhances insulin release in response to glucose via activation of its G protein-coupled receptor. This leads to stimulation of protein kinase A (PKA) and guanine nucleotide exchange factor exchange protein activated by cyclic-AMP (EPAC2). Additionally, acetylcholine release from parasympathetic nerves boosts insulin release through activation of the M2 muscarinic receptor, involving diacyl glycerol (DAG) and PKC. The role of sympathetic nerves in insulin secretion involves changes in adenylyl cyclase and cyclic adenosine monophosphate (cAMP) levels. α2-Adrenergic agonists inhibit insulin secretion, while β-adrenergic agonists stimulate it. Additionally, insulin/insulin-like growth factor 1 (IGF-1) receptor signaling and GLP-1 receptor (GLP-1R) signaling can positively regulate β-cell mass through transactivation of the epidermal growth factor receptor and stimulation of the insulin receptor substrate 2 (IRS-2) pathway. PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; ND6, NADH dehydrogenase subunit 6; TCA, tricarboxylic cycle; ER, endoplasmic reticulum; NEFA, non-esterified fatty acid; GABA, gamma-aminobutyric acid; GPR40, G-protein-coupled receptor 40.
Insulin sensitivity is highly dependent on the distribution of body fat, and it can vary markedly among lean individuals because of differences in their body fat distribution [22]. Lean individuals with a greater peripheral distribution of fat tend to have higher insulin sensitivity than those with a more central distribution of fat in the chest and abdominal area. The adipose tissue in these two locations (abdominal and subcutaneous fat) has distinct characteristics that result in different metabolic effects. Depending on the location, different adipocytes release specific amounts of protein [23]. Omental adipocytes, which are smaller than subcutaneous adipocytes, secrete larger amounts of adiponectin. This secretion has a stronger negative correlation with an individual’s body mass index than adiponectin derived from subcutaneous fat. More secretory proteins and proteins involved in energy production are encoded by intra-abdominal fat reserves. Despite the large amount of adiponectin secreted by intra-abdominal adipocytes, the subcutaneous fat reserves, which account for a larger proportion of body fat, contribute more significantly to adiponectin production [24].
PANCREATIC β-CELL DYSFUNCTION
Healthy β-cells actively respond to insulin resistance by modifying both their mass and function to maintain normal glucose levels. When β-cell dysfunction occurs, it leads to impaired glucose tolerance and abnormal fasting glucose levels, which consequently results in T2D [25].
Several observations have been reported regarding the magnitude of disruption of β-cells in T2D [26,27]. First, the β-cells in individuals with T2D, despite having insulin stores, are unable to secrete insulin rapidly in response to intravenous glucose intake. Second, compared to healthy subjects, a short-lasting burst of insulin is secreted by dysfunctional β-cells in response to non-glucose secretagogues. Third, it has been observed that there is approximately a 50% reduction in the normal functioning of β-cells in T2D. However, this cannot fully account for the damage to the cells’ secretory functioning, as the cells are already operating at 25% or less of their activity at the time of diabetes diagnosis [28]. As plasma NEFA levels increase, a progressive loss of β-cell function occurs. This is because higher plasma concentrations of NEFAs, as opposed to lower levels, are associated with abnormal glucose-stimulated insulin secretion and reduced insulin biosynthesis. Studies have reported that in vivo lipid infusion to elevate NEFA levels contributes to the development of insulin resistance, along with a slower counteracting response of β-cells in humans [29,30]. Therefore, NEFAs can be suggested as a critical factor linking insulin resistance and β-cell dysfunction in patients with T2D. Additionally, the lipotoxic effect derived from obesity may also contribute to the pathogenesis of diabetes, a phenomenon commonly referred to as “glucolipotoxicity” [31].
THE EFFECTS OF TAURINE ON OBESITY: ANIMAL STUDIES
Taurine has been linked to several anti-obesity mechanisms in various animal models. In Institute of Cancer Research (ICR) mice models fed a high-fat diet, the inclusion of 2% taurine in their diet led to a significant decrease in body mass. This decrease was achieved through the reduction of adipogenic gene expression (specifically PPAR-α and CCAAT/enhancer-binding protein C/EPR-α [CEBP-β]) in white adipose tissue [32]. In male obese rats, taurine supplementation resulted in a significant reduction in retroperitoneal and perigonadal fat pads, as well as overall body weight. This reduction was attributed to the lower caloric intake observed in the taurine-supplemented group [33].
Modulations in energy metabolism have been observed in C57BL/6J mice subjected to a 5% taurine treatment, with taurine causing a decrease in body weight, body fat percentage, and adipocyte size [34]. These anti-obesity effects are attributed to an increase in resting energy oxygen consumption, which is facilitated by an increase in the expression of genes associated with energy metabolism in white adipose tissues. These genes include nuclear respiratory factor 2 α lipoprotein lipase, acyl-CoA oxidase, acyl-CoA synthetase, β-subunit of adenosine triphosphate (ATP) synthetase, and medium-chain acyl-CoA dehydrogenase (MCAD).
Rats with monosodium glutamate (MSG)-induced obesity that were supplemented with taurine (2.5%) had a decrease in proportion of perigonadal and retroperitoneal fat pad mass due to a reduction in the increased diameter and size of adipocytes [35]. Furthermore, MSG injection led to increased phosphorylation of Ik-βα in the adipose tissue, a process that was reversed by taurine intake. In another study involving C57BL/6J mice fed a high-fat diet, a 5% taurine intake resulted in a reduction in mesenteric, subcutaneous, retroperitoneal, and epididymal white adipose tissue [36]. Immunohistochemistry revealed that taurine supplementation reduced staining for the M1 marker CD11c and increased staining for the M2 marker IL-10. This was accompanied by an increased content of CD206+ cells, Ym1 markers, and a reduction of CD11c+ cells, along with IL-10 in the epididymal white adipose tissue extracted stromal vascular fractions. In addition to an increase in IL-10 protein levels in the white adipose tissue, in vitro-grown bone marrow-derived macrophage cells exhibited an enhanced expression of M2 markers, including Ym1, CD206, and macrophage galactose-type lectin-1.
Isoproterenol, a selective agonist for β-adrenergic receptors known to increase lipolysis in adipocytes, was found to enhance lipolysis in isolated rat adipocyte cells when supplemented with taurine [37]. Furthermore, in C3H10T1/2 adipocytes, there was an observed increase in the expression of energy expenditure and thermogenic genes (peroxisome proliferator-activated receptor-γ coactivator 1-α [PGC1α], uncoupling protein 1 [UCP1], carnitine palmitoyl transferase-1β [Cpt1β]). Taurine also elevated the protein-level expression of PGC1α and UCP1 in C2H1OT1/2 adipocyte cells [34].
THE EFFECTS OF TAURINE ON DIABETES: ANIMAL STUDIES
Previous research has explored the potential role of taurine in mitigating the effects of diabetes. In one study, male C57BL/6J mice that received taurine supplementation (5% in drinking water) demonstrated an improved glucose tolerance level, as determined by an intraperitoneal glucose tolerance test [38]. Another study, conducted on male Wistar rats fed a high-fructose diet with taurine supplementation, found a significant reduction in glycated hemoglobin and proteins, fructose amines, and plasma glucose levels [39]. These studies suggest that taurine may positively influence glucose metabolism in animal models. Furthermore, in male Otsuka Long-Evans Tokushima Fatty (OLETF) rats and Sprague-Dawley rats, taurine supplementation significantly increased glucose utilization, thereby reducing plasma glucose levels [40]. These findings further indicate that taurine may have beneficial effects on glucose metabolism in diabetic animal models.
In a study involving male Wistar rats, taurine supplementation was found to enhance insulin signaling in the liver by increasing the rate of utilization of infused glucose and reducing hepatic glucose production [41]. The presence of taurine was also linked to a decrease in the phosphorylation of c-Jun N-terminal kinase (JNK) and serine phosphorylation of the insulin receptor substrate 1 and 2 (IRS-1 and -2). Simultaneously, there was an increase in the phosphorylation of IRS-1 and -2 tyrosine and serine 473 of Akt in the liver, suggesting improved insulin signaling. Furthermore, taurine was found to increase the phosphorylation of phosphatase and tensin homolog in conjunction with Akt in the liver of C57BL/6J mice, indicating an enhancement of the insulin signaling pathway [42].
In vivo studies have demonstrated an enhancement in glucose metabolism following taurine consumption. When male Wistar rats were given a 2% taurine supplement in their drinking water, it prevented the increase in glucose levels typically associated with a high-fructose diet, thereby improving glucose tolerance [43]. This high-fructose diet also influenced the activity of enzymes involved in liver glucose metabolism, including glucose 6-phosphatase, fructose 1,6-bisphosphatase, pyruvate kinase, and hexokinase. Furthermore, the high-fructose diet caused changes in the activity of protein tyrosine kinase and protein tyrosine phosphatase in the liver, which taurine was able to neutralize. Since the activity of these enzymes impacts insulin signaling, the findings suggest that taurine may help regulate blood glucose levels by enhancing insulin signaling.
In another study, male C57BL/6J mice were given arsenic trioxide (As2O3) in their drinking water, with or without taurine supplementation (250 mg/kg) [44]. The exposure to As2O3 resulted in impaired glucose tolerance, which was reversed by taurine. This reversal was accompanied by a decrease in the expression of genes associated with gluconeogenesis, such as phosphoenol pyruvate carboxykinase, fructose 1,6 bisphosphate, and PGC1α. Conversely, the process of glycolysis was enhanced, as evidenced by the upregulated expression of L-type pyruvate kinase and glucokinase (GCK) genes in the mice’s livers. These findings suggest that taurine may have a beneficial effect on liver glucose metabolism during insulin resistance. Furthermore, taurine supplementation increased the protein expression levels of PPAR-γ and phosphorylated Akt in the mouse liver, potentially further improving hepatic glucose metabolism.
Taurine has been shown to offer protective benefits to pancreatic β-cells in various studies. For instance, male Swiss mice supplemented with 5% taurine demonstrated inhibited glucose and insulin levels [45]. A high-fat diet led to a significant increase in both β-cell mass and islet mass, which was counteracted by taurine intake. In a study involving isolated β-cells that overexpressed UCP2, treatment with taurine (3 mM) resulted in a significant enhancement of insulin secretion in response to glucose [46]. In the pancreatic β-cells, prolonged exposure to acids or glucose has been associated with increased UCP2 expression, which in turn has been linked to impaired insulin secretion. Additionally, the levels of methyl pyruvate-induced calcium content were increased in mitochondria treated with taurine in the overexpressed UCP2 β-cells. Therefore, it can be inferred that taurine may enhance glucose sensitivity by increasing calcium influx into the mitochondria via the calcium uniporter, thereby improving mitochondrial metabolism in the β-cells.
MECHANISMS
Antioxidation
Taurine mitigates and counteracts the production of reactive oxygen species (ROS) by indirectly alleviating heightened oxidative stress. This is achieved through several methods, one of which includes enhancing the activity of anti-oxidative enzymes such as catalase (CAT), glutathione peroxidase (GSH-Px), and superoxide dismutase. Additionally, taurine contributes to the reduction of lipid peroxidation and the formation of carbonyl proteins, and it inhibits the activity of protein kinase C and xanthine oxidase [47,48]. It also hinders the formation of advanced glycated end products and suppresses the expression and activity of co-enzyme II (nicotinamide-adenine dinucleotide phosphate [NADPH] II), oxidase C(47phox)/cytochrome P40 CYP2E1 [49]. Exposure of isolated cardiomyocytes to β-alanine in the medium (an inhibitor of the taurine transporter) results in increased oxidative stress in the mitochondria of cells. This is due to the generation of super oxides, inactivation of oxidoreductase, and oxidation of glutathione, leading to a 45% decrease in overall tissue content [50]. This reduction in taurine content subsequently causes a decrease in the production of NADH dehydrogenase subunit 5 and 6 (ND5 and ND6) subunits of the mitochondrial respiratory chain. This results in defective complexes I and II, which in turn diminishes the potential transfer of electrons in the respiratory chain, leading to an increase in ROS.
A 30% reduction in serum glucose levels was observed in rats with diabetes induced via alloxan (ALX) and treated with 1% taurine (in their drinking water) [12]. This was accompanied by an improvement in renal gluconeogenesis. Taurine treatment also increased renal glutathione reductase and CAT activities, restored the ratio of GSH to glutathione disulfide, reduced albuminuria and glomerulopathy, and inhibited the accumulation of ROS and free hydroxyl ions in the liver, serum, and kidney cortex. These findings underscore the crucial role of taurine as an antioxidant in the treatment of diabetes and diabetic nephropathy. The potential protective effects of taurine and the mechanisms behind them, as detailed in various studies, are summarized, and presented in Tables 1 [45,51-56] and 2 [57-61].
Table 1.
Studies on the Effects of Taurine and the Mechanisms behind the Action of Taurine
| Study | Activity | Mechanism |
|---|---|---|
| Kulakowski et al. (1984) [52], Wu et al. (2010) [51] | Enhancement of insulin sensitivity | Insulin resistance can be caused by changes in phosphorylation states of certain proteins, such as IRS-1, IRS-2, Akt, and JNK-1 in peripheral tissues. Taurine regulates these changes through direct interactions with insulin receptors. |
| Carneiro et al. (2009) [45], Park et al. (2004) [53] | Inducing insulin secretion | By increasing the expression of genes involved in insulin secretion and/or by blocking ATP-sensitive potassium channels, insulin production is stimulated. |
| Jong et al. (2012) [54] | Anti-oxidation | Taurine protects the mitochondria from excessive superoxide production by binding to the uridine moiety of mitochondrial tRNA Leu. |
| Park et al. (1993) [55], Liu et al. (2002) [56] | Anti-inflammation | Taurine has been found to suppress the secretion of cytokines that are related to diabetes, including TNF-α and MCP-1. These cytokines are known to play a role in the development and progression of diabetes. By suppressing their secretion, taurine helps to reduce the risk or severity of diabetes. |
IRS, insulin receptor substrate; JNK-1, c-Jun N-terminal kinase1; ATP, adenosine triphosphate; TNF-α, tumor necrosis factor-alpha; MCP-1, monocyte chemoattractant protein 1.
Table 2.
Studies Summarizing the Effects of Taurine and the Mechanisms Underlying Those Effects
| Effect | Underlying mechanism |
|---|---|
| Antioxidative effects | Upregulation of cellular antioxidant defense system by enhancement of anti-oxidative enzymes, such as hepatic thioredoxin reductase activity [57], superoxide dismutase and glutathione peroxidase activity [58]. Inhibition of mitochondrial stress by disruption of events causing intracellular Ca2+ overload. |
| Anti-inflammatory effects | Scavenging of hypochlorous acid and inhibition of the production of nitric oxide and TNF-α [59]. |
| Energy metabolism | Reduction of the NADH/NAD+ ratio during glycolysis, thus activating complex I and NADH-sensitive enzymes [60]. |
| Pancreatic β-cell function | Taurine inactivates ATP-sensitive K+ channels, causing intracellular Ca2+ overload that results in exocytosis of insulin molecules, thereby enhancing insulin secretion and availability [61]. |
TNF-α, tumor necrosis factor-alpha; NADH/NAD, reduced and oxidized forms of nicotinamide adenine dinucleotide; ATP, adenosine triphosphate.
Anti-inflammation
Diabetes is characterized not only by elevated blood glucose levels but also by an underlying inflammatory process. Lymphocytes produce inflammatory cytokines that can destroy insulin-producing β-cells and increase the level of inflammatory markers in individuals with diabetes and insulin resistance [62]. Neutrophils produce hypochlorous acid, which can cause oxidative stress and activate tyrosine kinase, leading to the formation of inflammatory mediators through a signaling cascade. Taurine, found in high amounts in neutrophils and monocytes, reacts with hypochlorous acid to form taurine chloramines. These chloramines reduce the inflammatory cytokine mediators due to their anti-inflammatory and anti-oxidative effects, and they also decrease the activity of nuclear factor kappa β, resulting in reduced secretion of nitric oxide, TNF, prostaglandin, and interleukins [63]. Li et al. [13] investigated the impact of taurine supplementation in mothers and neonate Wistar rats on inflammation and lipid metabolism [13,64]. Their results indicated that taurine supplementation was able to normalize maternal plasma glucose levels and TNF-α, as well as improve liver function. In another study, Lin et al. [36] found that taurine treatment in C57BL/6J mice reduced the infiltration of pro-inflammatory macrophages in adipose tissue, while promoting the anti-inflammatory M2-like phenotype.
ENHANCING PANCREATIC β-CELL FUNCTION AND INSULIN SECRETION
Taurine supplementation has been demonstrated to decrease necrosis of islet β-cells and enhance both the cell count and secretory granules in diabetic rats and mice treated with streptozotocin (STZ) [12]. Yao et al. [65] reported similar findings, revealing reduced mitochondrial and endoplasmic reticulum damage in 1%–3% of the taurine-supplemented diabetic mice group. This resulted in a significant increase in insulin levels within the β-cells. Furthermore, the induction of IL-β, TNF-α, and interferon-γ into isolated islet cells of rats led to a notable increase in apoptosis, which was counteracted by taurine intake through the modulation of the ratio of pro-apoptotic to anti-apoptotic gene (Bax, B-cell lymphoma-extra-large [Bcl-xl]). These findings suggest that the protective effect of taurine on pancreatic β-cells may be attributed to its ability to alleviate inflammation and destruction of islet cells induced by STZ.
Oprescu et al. [66] conducted an in vitro study to evaluate the impact of taurine on insulin secretion in Wistar rats. The results showed that the infusion of oleate decreased insulin levels in response to hyperglycemic clamps and diminished the secretion of insulin stimulated by glucose. Both outcomes were linked to oxidative stress in isolated islets. However, the co-infusion of taurine mitigated these adverse effects. In a similar vein, Ribeiro et al. [67] examined the effects of taurine on isolated islets of mice. They found improved glucose tolerance and enhanced insulin sensitivity in mice, as demonstrated by increased insulin secretion from their islets in response to high glucose levels. However, there was no difference in ATP levels, glucose transport protein-2, or GCK (liver glucose kinase) protein expression between the groups. When compared to the control mice islets, the taurine-supplemented islets exhibited a significant increase in the expression of l-type voltage-sensitive Ca2+ channels and Ca2+ uptake. These results suggest that taurine may influence insulin secretion by regulating intracellular calcium levels. Taurine achieves this by blocking ATP-sensitive potassium channels, disrupting the interaction between the sulfonyl urea receptor-1 (SUR-1) and glipalamide. This leads to cell depolarization and the opening of calcium-dependent voltage channels, causing an influx of extracellular calcium. When the calcium concentration reaches a critical level, insulin is secreted through the process of exocytosis by large, dense core vesicles.
Glucose homeostasis is maintained by modulating the expression of genes involved in insulin secretion, a process influenced by taurine. When stimulated by glucose, the islet cells of mice demonstrated a significantly higher insulin content after taurine treatment. The expression of genes that regulate insulin, such as SUR-1, GCK, glucose transporter 2 (GLUT-2), and pancreatic and duodenal homeobox 1 (PDX-1), was also elevated in these treated mice [68]. PDX-1 serves as a key regulator of endocrine function in islet cells, modulating the gene expression of GLUT-2 and GCK, which are insulin-regulating genes. Furthermore, PDX-1 also functions as a transcription factor for the expression of the insulin gene.
ENHANCING INSULIN SENSITIVITY
Ribeiro et al. [68] reported that diabetic mice and rats, when given a 2% taurine intake in their drinking water, exhibited increased insulin sensitivity and improved glucose tolerance. Haber et al.’s [69] experiment on non-diabetic rats demonstrated that co-infusing taurine with glucose reduced insulin-stimulated glucose uptake levels and 4-hydroxy nonanal content. Furthermore, in non-diabetic overweight or obese men, oral taurine intake reduced oxidative stress and improved lipid-infused β-cell decompensation and insulin resistance through an increase in IRS phosphorylation. Carneiro et al. [45] demonstrated similar effects of basal IRS phosphorylation in the skeletal muscle and liver of mice. This effect facilitates the binding of insulin to its receptors and glucose metabolism, resulting in improved intraperitoneal glucose tolerance. In a study by Wu et al. [51], normal rats were infused with a mixture of intralipid and heparin (IH) to elevate free fatty acid levels in the plasma. This IH intake increased JNK-1 activity and induced serine phosphorylation of IRS-1 and -2, leading to insulin resistance in the liver. Therefore, these findings suggest that taurine may improve insulin resistance through the inhibition of the JNK pathway and the enhancement of insulin signaling.
CONCLUSIONS
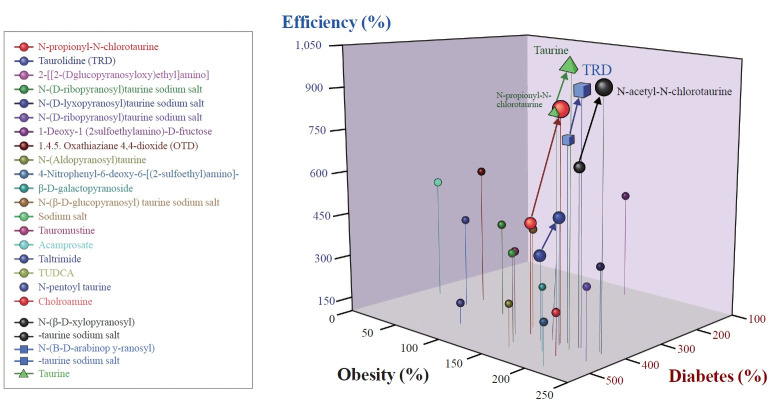
In conclusion, this review highlights the strong link between obesity, insulin resistance, and the development of T2D, in which β-cell dysfunction plays a significant role. A highly remarkable finding from numerous in vivo and in vitro studies is the mitigating effect of taurine on metabolic syndrome, as detailed in this review. Taurine prevents obesity primarily through three mechanisms: increasing energy expenditure by enhancing factors involved in fatty acid oxidation, decreasing lipogenesis by reducing postprandial glucose oxidation, and modifying the energy ratio used for glycogen synthesis and lipogenesis. Furthermore, taurine prevents hypercholesterolemia by facilitating the bioconversion of cholesterol to bile acids, promoting its excretion through feces, inhibiting bile acids absorption from the ileum, reducing very low-density lipoprotein secretion from the liver, and enhancing cholesterol clearance from the bloodstream by upregulating low-density lipoprotein receptor gene expression and binding capacity. This leads to an increase in low-density lipoprotein cholesterol and mitigates diabetes mellitus by decreasing insulin resistance, boosting insulin production, protecting pancreatic cells, and through anti-inflammatory and anti-oxidative effects. The comprehensive beneficial efficiency of taurine and its derivatives on obesity and T2D are depicted in Fig. 2. However, additional research is required to further elucidate the precise mechanisms underlying taurine’s beneficial effects in the prevention and management of T2D.
Fig. 2.
Comparative analysis of ameliorative effects caused by taurine and its derivatives. Top to bottom N-propionyl N-cholrotaurine, taurolidine (TRD), 2 (D-glucopyranosyl) ethyl amino, N-(D-ribopyranosyl) taurine sodium salt, N (D-lyxopyronosyl) taurine sodium salt, N- (D-ribopyranosyl) taurine sodium salt, 1-deoxy (2 sulphoethylamine)-D-fructose, 1.4.5 oxathiaziane 4,4-dioxide (OTD), N-(aldopyranosyl) taurine, 4 nitrophenyl 1-6-deoxy-6-[2 sulphoethyl amino], β-D-galactopyranoside, N-(β-D-glucopyranosyl) taurine sodium salt, tauronustine, acamprostate, taltrimide, tauroursodeoxycholic acid sodium salt (TUCDA), N-phenoyl taurine, chloramine, N-(β-dxylopyranosyl) taurine sodium salt, N-(β-D-arabinopyranosyl) taurine sodium salt, taurine.
Despite the promising results obtained in preclinical studies, the efficacy of taurine supplementation for the management of diabetes in humans remains unclear. Although several animal studies have reported beneficial effects of taurine supplementation on glucose homeostasis, insulin secretion, and sensitivity, the evidence from human studies remains inconsistent. Therefore, it is important to conduct more adequately powered, double-blinded, randomized, and controlled trials to determine the effects of taurine supplementation on diabetes management in humans. Furthermore, the mechanisms underlying the potential beneficial effects of taurine on diabetes should be further elucidated. Therefore, caution should be exercised when interpreting the results of taurine supplementation in the context of diabetes management until more robust clinical evidence is available.
Acknowledgments
This research is funded by the Financial Program for Self-Directed Research Capacity in 2022.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014;7:45–8. doi: 10.4066/AMJ.2013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 4.Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes. 2011;60:918–24. doi: 10.2337/db10-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uyanga VA, Oke EO, Amevor FK, Zhao J, Wang X, Jiao H, et al. Functional roles of taurine, L-theanine, L-citrulline, and betaine during heat stress in poultry. J Anim Sci Biotechnol. 2022;13:23. doi: 10.1186/s40104-022-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakaria M, Azam S, Haque ME, Jo SH, Uddin MS, Kim IS, et al. Taurine and its analogs in neurological disorders: focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019;24:101223. doi: 10.1016/j.redox.2019.101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheri FH, Dawood H, Hassan JK, Aljazaeari QA. Study the effects of taurine oral supplement used for type 2 diabetic patients on body weight; glycemic control and some bone mineralization biochemical markers. Bull Fac Pharm Cairo Univ. 2021;59:27–32. [Google Scholar]
- 8.Tenner TE, Jr, Zhang XJ, Lombardini JB. Hypoglycemic effects of taurine in the alloxan-treated rabbit, a model for type 1 diabetes. Adv Exp Med Biol. 2003;526:97–104. doi: 10.1007/978-1-4615-0077-3_13. [DOI] [PubMed] [Google Scholar]
- 9.Brons C, Spohr C, Storgaard H, Dyerberg J, Vaag A. Effect of taurine treatment on insulin secretion and action, and on serum lipid levels in overweight men with a genetic predisposition for type II diabetes mellitus. Eur J Clin Nutr. 2004;58:1239–47. doi: 10.1038/sj.ejcn.1601955. [DOI] [PubMed] [Google Scholar]
- 10.Imae M, Asano T, Murakami S. Potential role of taurine in the prevention of diabetes and metabolic syndrome. Amino Acids. 2014;46:81–8. doi: 10.1007/s00726-012-1434-4. [DOI] [PubMed] [Google Scholar]
- 11.Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17:330–46. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Guo J, Zhang Y, Zhang J. The beneficial effects of taurine in preventing metabolic syndrome. Food Funct. 2016;7:1849–63. doi: 10.1039/c5fo01295c. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Reynolds CM, Sloboda DM, Gray C, Vickers MH. Effects of taurine supplementation on hepatic markers of inflammation and lipid metabolism in mothers and offspring in the setting of maternal obesity. PLoS One. 2013;8:e76961. doi: 10.1371/journal.pone.0076961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernon RG. Lipid metabolism during lactation: a review of adipose tissue-liver interactions and the development of fatty liver. J Dairy Res. 2005;72:460–9. doi: 10.1017/S0022029905001299. [DOI] [PubMed] [Google Scholar]
- 15.Abranches MV, Oliveira FC, Conceicao LL, Peluzio MD. Obesity and diabetes: the link between adipose tissue dysfunction and glucose homeostasis. Nutr Res Rev. 2015;28:121–32. doi: 10.1017/S0954422415000098. [DOI] [PubMed] [Google Scholar]
- 16.Badoiu SC, Miricescu D, Stanescu-Spinu II, Ripszky Totan A, Badoiu SE, Costagliola M, et al. Glucose metabolism in burns: what happens? Int J Mol Sci. 2021;22:5159. doi: 10.3390/ijms22105159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–70. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 18.Ni Y, Ni L, Zhuge F, Xu L, Fu Z, Ota T. Adipose tissue macrophage phenotypes and characteristics: the key to insulin resistance in obesity and metabolic disorders. Obesity (Silver Spring) 2020;28:225–34. doi: 10.1002/oby.22674. [DOI] [PubMed] [Google Scholar]
- 19.Mendez-Sanchez N, Valencia-Rodriguez A, Coronel-Castillo C, Vera-Barajas A, Contreras-Carmona J, Ponciano-Rodriguez G, et al. The cellular pathways of liver fibrosis in non-alcoholic steatohepatitis. Ann Transl Med. 2020;8:400. doi: 10.21037/atm.2020.02.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7:216. doi: 10.1038/s41392-022-01073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suba K. London: Imperial College London; 2020. Longitudinal imaging of pancreatic islets transplanted into the anterior chamber of the eye [dissertation] [Google Scholar]
- 22.van Vliet S, Koh HE, Patterson BW, Yoshino M, LaForest R, Gropler RJ, et al. Obesity is associated with increased basal and postprandial β-cell insulin secretion even in the absence of insulin resistance. Diabetes. 2020;69:2112–9. doi: 10.2337/db20-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–68. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engin A. Adiponectin-resistance in obesity. Adv Exp Med Biol. 2017;960:415–41. doi: 10.1007/978-3-319-48382-5_18. [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–9. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 26.Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16:349–62. doi: 10.1038/s41574-020-0355-7. [DOI] [PubMed] [Google Scholar]
- 27.Cho JH, Kim JW, Shin JA, Shin J, Yoon KH. β-Cell mass in people with type 2 diabetes. J Diabetes Investig. 2011;2:6–17. doi: 10.1111/j.2040-1124.2010.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devedjian JC, George M, Casellas A, Pujol A, Visa J, Pelegrin M, et al. Transgenic mice overexpressing insulin-like growth factor-II in beta cells develop type 2 diabetes. J Clin Invest. 2000;105:731–40. doi: 10.1172/JCI5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98:2133–223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva Rosa SC, Nayak N, Caymo AM, Gordon JW. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol Rep. 2020;8:e14607. doi: 10.14814/phy2.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic syndrome pathophysiology and predisposing factors. Int J Sports Med. 2021;42:199–214. doi: 10.1055/a-1263-0898. [DOI] [PubMed] [Google Scholar]
- 32.Kim KS, Jang MJ, Fang S, Yoon SG, Kim IY, Seong JK, et al. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids. 2019;51:245–54. doi: 10.1007/s00726-018-2659-7. [DOI] [PubMed] [Google Scholar]
- 33.Wen C, Li F, Zhang L, Duan Y, Guo Q, Wang W, et al. Taurine is involved in energy metabolism in muscles, adipose tissue, and the liver. Mol Nutr Food Res. 2019;63:e1800536. doi: 10.1002/mnfr.201800536. [DOI] [PubMed] [Google Scholar]
- 34.Guo YY, Li BY, Peng WQ, Guo L, Tang QQ. Taurine-mediated browning of white adipose tissue is involved in its antiobesity effect in mice. J Biol Chem. 2019;294:15014–24. doi: 10.1074/jbc.RA119.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caetano LC, Bonfleur ML, Ribeiro RA, Nardelli TR, Lubaczeuski C, do Nascimento da Silva J, et al. Taurine supplementation regulates Iκ-Bα protein expression in adipose tissue and serum IL-4 and TNF-α concentrations in MSG obesity. Eur J Nutr. 2017;56:705–13. doi: 10.1007/s00394-015-1114-8. [DOI] [PubMed] [Google Scholar]
- 36.Lin S, Hirai S, Yamaguchi Y, Goto T, Takahashi N, Tani F, et al. Taurine improves obesity-induced inflammatory responses and modulates the unbalanced phenotype of adipose tissue macrophages. Mol Nutr Food Res. 2013;57:2155–65. doi: 10.1002/mnfr.201300150. [DOI] [PubMed] [Google Scholar]
- 37.Leroux M, Lemery T, Boulet N, Briot A, Zakaroff A, Bouloumie A, et al. Effects of the amino acid derivatives, βhydroxy-β-methylbutyrate, taurine, and N-methyltyramine, on triacylglycerol breakdown in fat cells. J Physiol Biochem. 2019;75:263–73. doi: 10.1007/s13105-019-00677-5. [DOI] [PubMed] [Google Scholar]
- 38.Batista TM, Ribeiro RA, da Silva PM, Camargo RL, Lollo PC, Boschero AC, et al. Taurine supplementation improves liver glucose control in normal protein and malnourished mice fed a high-fat diet. Mol Nutr Food Res. 2013;57:423–34. doi: 10.1002/mnfr.201200345. [DOI] [PubMed] [Google Scholar]
- 39.Rafiee Z, Garcia-Serrano AM, Duarte JM. Taurine supplementation as a neuroprotective strategy upon brain dysfunction in metabolic syndrome and diabetes. Nutrients. 2022;14:1292. doi: 10.3390/nu14061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bae M, Ahmed K, Yim JE. Beneficial effects of taurine on metabolic parameters in animals and humans. J Obes Metab Syndr. 2022;31:134–46. doi: 10.7570/jomes21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saad SY, Al-Rikabi AC. Protection effects of taurine supplementation against cisplatin-induced nephrotoxicity in rats. Chemotherapy. 2002;48:42–8. doi: 10.1159/000048587. [DOI] [PubMed] [Google Scholar]
- 42.Cappelli AP, Zoppi CC, Barbosa-Sampaio HC, Costa JM, Jr, Protzek AO, Morato PN, et al. Taurine-induced insulin signalling improvement of obese malnourished mice is associated with redox balance and protein phosphatases activity modulation. Liver Int. 2014;34:771–83. doi: 10.1111/liv.12291. [DOI] [PubMed] [Google Scholar]
- 43.Larsen LH, Orstrup LK, Hansen SH, Grunnet N, Quistorff B, Mortensen OH. The effect of long-term taurine supplementation and fructose feeding on glucose and lipid homeostasis in Wistar rats. Adv Exp Med Biol. 2013;776:39–50. doi: 10.1007/978-1-4614-6093-0_5. [DOI] [PubMed] [Google Scholar]
- 44.Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018;9:946. doi: 10.1038/s41419-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carneiro EM, Latorraca MQ, Araujo E, Beltra M, Oliveras MJ, Navarro M, et al. Taurine supplementation modulates glucose homeostasis and islet function. J Nutr Biochem. 2009;20:503–11. doi: 10.1016/j.jnutbio.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Han J, Bae JH, Kim SY, Lee HY, Jang BC, Lee IK, et al. Taurine increases glucose sensitivity of UCP2-overexpressing beta-cells by ameliorating mitochondrial metabolism. Am J Physiol Endocrinol Metab. 2004;287:E1008–18. doi: 10.1152/ajpendo.00008.2004. [DOI] [PubMed] [Google Scholar]
- 47.Wu G, San J, Pang H, Du Y, Li W, Zhou X, et al. Taurine attenuates AFB1-induced liver injury by alleviating oxidative stress and regulating mitochondria-mediated apoptosis. Toxicon. 2022;215:17–27. doi: 10.1016/j.toxicon.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Zong X, Wu G, Lin S, Feng Y, Hu J. Taurine increases testicular function in aged rats by inhibiting oxidative stress and apoptosis. Amino Acids. 2015;47:1549–58. doi: 10.1007/s00726-015-1995-0. [DOI] [PubMed] [Google Scholar]
- 49.Roy A, Sil PC. Tertiary butyl hydroperoxide induced oxidative damage in mice erythrocytes: protection by taurine. Pathophysiology. 2012;19:137–48. doi: 10.1016/j.pathophys.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Jong CJ, Azuma J, Schaffer SW. Role of mitochondrial permeability transition in taurine deficiency-induced apoptosis. Exp Clin Cardiol. 2011;16:125–8. [PMC free article] [PubMed] [Google Scholar]
- 51.Wu N, Lu Y, He B, Zhang Y, Lin J, Zhao S, et al. Taurine prevents free fatty acid-induced hepatic insulin resistance in association with inhibiting JNK1 activation and improving insulin signaling in vivo. Diabetes Res Clin Pract. 2010;90:288–96. doi: 10.1016/j.diabres.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Kulakowski EC, Maturo J. Hypoglycemic properties of taurine: not mediated by enhanced insulin release. Biochem Pharmacol. 1984;33:2835–8. doi: 10.1016/0006-2952(84)90204-1. [DOI] [PubMed] [Google Scholar]
- 53.Park EJ, Bae JH, Kim SY, Lim JG, Baek WK, Kwon TK, et al. Inhibition of ATP-sensitive K+ channels by taurine through a benzamido-binding site on sulfonylurea receptor 1. Biochem Pharmacol. 2004;67:1089–96. doi: 10.1016/j.bcp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Jong CJ, Azuma J, Schaffer S. Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids. 2012;42:2223–32. doi: 10.1007/s00726-011-0962-7. [DOI] [PubMed] [Google Scholar]
- 55.Park E, Quinn MR, Wright CE, Schuller-Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J Leukoc Biol. 1993;54:119–24. doi: 10.1002/jlb.54.2.119. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Quinn MR. Chemokine production by rat alveolar macrophages is inhibited by taurine chloramine. Immunol Lett. 2002;80:27–32. doi: 10.1016/s0165-2478(01)00291-7. [DOI] [PubMed] [Google Scholar]
- 57.Yildirim Z, Kilic N, Ozer C, Babul A, Take G, Erdogan D. Effects of taurine in cellular responses to oxidative stress in young and middle-aged rat liver. Ann N Y Acad Sci. 2007;1100:553–61. doi: 10.1196/annals.1395.061. [DOI] [PubMed] [Google Scholar]
- 58.Vohra BP, Hui X. Taurine protects against carbon tetrachloride toxicity in the cultured neurons and in vivo. Arch Physiol Biochem. 2001;109:90–4. doi: 10.1076/apab.109.1.90.4287. [DOI] [PubMed] [Google Scholar]
- 59.Kim C, Cha YN. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids. 2014;46:89–100. doi: 10.1007/s00726-013-1545-6. [DOI] [PubMed] [Google Scholar]
- 60.Schaffer SW, Shimada-Takaura K, Jong CJ, Ito T, Takahashi K. Impaired energy metabolism of the taurine-deficient heart. Amino Acids. 2016;48:549–58. doi: 10.1007/s00726-015-2110-2. [DOI] [PubMed] [Google Scholar]
- 61.L’Amoreaux WJ, Cuttitta C, Santora A, Blaize JF, Tachjadi J, El Idrissi A. Taurine regulates insulin release from pancreatic beta cell lines. J Biomed Sci. 2010;17(Suppl 1):S11. doi: 10.1186/1423-0127-17-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167:228–56. doi: 10.1016/j.trsl.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Murakami S. The physiological and pathophysiological roles of taurine in adipose tissue in relation to obesity. Life Sci. 2017;186:80–6. doi: 10.1016/j.lfs.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Li M, Reynolds CM, Sloboda DM, Gray C, Vickers MH. Maternal taurine supplementation attenuates maternal fructose-induced metabolic and inflammatory dysregulation and partially reverses adverse metabolic programming in offspring. J Nutr Biochem. 2015;26:267–76. doi: 10.1016/j.jnutbio.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Yao HT, Lin P, Chang YW, Chen CT, Chiang MT, Chang L, et al. Effect of taurine supplementation on cytochrome P450 2E1 and oxidative stress in the liver and kidneys of rats with streptozotocin-induced diabetes. Food Chem Toxicol. 2009;47:1703–9. doi: 10.1016/j.fct.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 66.Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, et al. Free fatty acid-induced reduction in glucosestimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes. 2007;56:2927–37. doi: 10.2337/db07-0075. [DOI] [PubMed] [Google Scholar]
- 67.Ribeiro RA, Vanzela EC, Oliveira CA, Bonfleur ML, Boschero AC, Carneiro EM. Taurine supplementation: involvement of cholinergic/phospholipase C and protein kinase A pathways in potentiation of insulin secretion and Ca2+ handling in mouse pancreatic islets. Br J Nutr. 2010;104:1148–55. doi: 10.1017/S0007114510001820. [DOI] [PubMed] [Google Scholar]
- 68.Ribeiro RA, Bonfleur ML, Amaral AG, Vanzela EC, Rocco SA, Boschero AC, et al. Taurine supplementation enhances nutrient-induced insulin secretion in pancreatic mice islets. Diabetes Metab Res Rev. 2009;25:370–9. doi: 10.1002/dmrr.959. [DOI] [PubMed] [Google Scholar]
- 69.Haber CA, Lam TK, Yu Z, Gupta N, Goh T, Bogdanovic E, et al. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. Am J Physiol Endocrinol Metab. 2003;285:E744–53. doi: 10.1152/ajpendo.00355.2002. [DOI] [PubMed] [Google Scholar]