Fig 1.
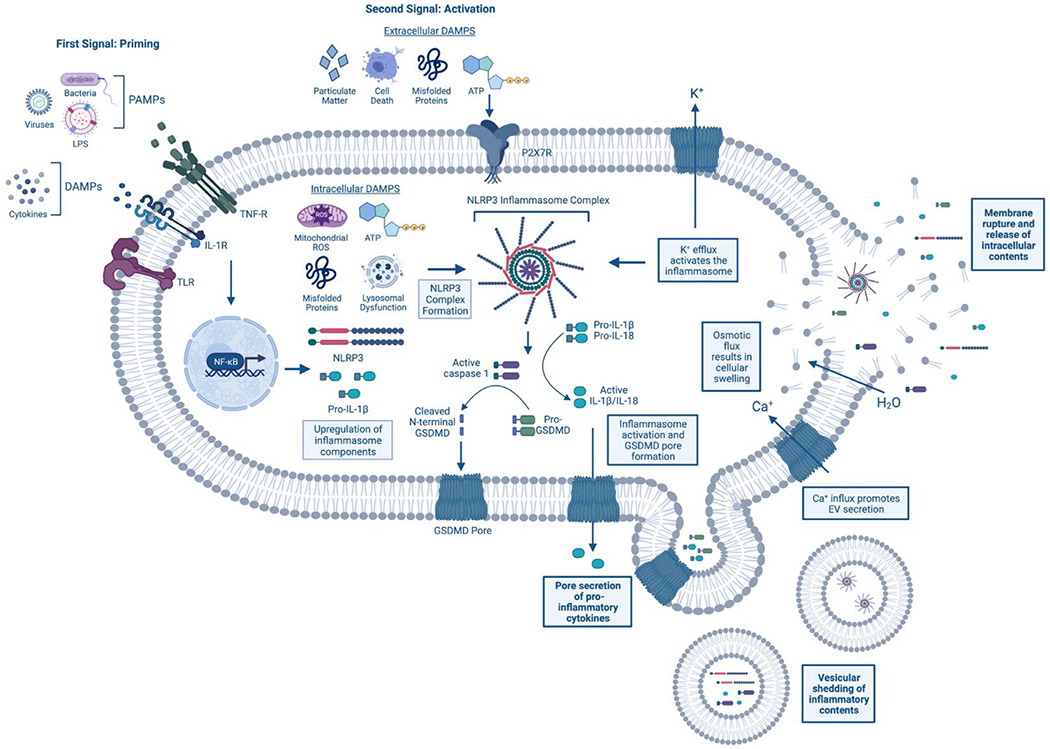
Mechanism of inflammasome activation and pyroptosis. The NLRP3 inflammasome is activated in a 2-step fashion. First, priming occurs at surface membrane receptors. This can include recognition of pathogen-associated molecular patterns (PAMPS) (eg, viruses and bacteria) at Toll-like receptors (TLRs) and/or danger-associated molecular patterns (DAMPs) (eg, cytokines) at the IL-1 receptor (IL-1R) or TNF-a receptor (TNF-R) for example. Following receptor activation, the transcription factor NF-KB translocates to the nucleus where it causes upregulation of inflammasome components, such as NLRP3 and pro-IL-1B. A secondary stimulus is necessary to cause inflammasome complex formation and activation. This second trigger can be either intra- and extra-cellular. Some common stimuli include both intra- and extra-cellular ATP and misfolded proteins, extracellular particulate matter (eg, silica, uric acid crystals) and components of cellular demise, and intracellular mitochondrial reactive oxygen species (ROS) and lysosomal dysfunction. After NLRP3 inflammasome complex formation, the pro-caspase-1 enzyme is cleaved into its active, mature form and can cleave both pro-inflammatory cytokines, IL-1B and IL-18, and the effector protein of pyroptosis, Gasdermin-D (GSDMD). Once cleaved, the N-terminal fragments of GSDMD form pores in the plasma membrane allowing for the extracellular release of the mature cytokines IL-1B and IL-18. Further, these pores promote osmotic and ionic flux, resulting in cellular swelling and the efflux of potassium (K+) and the influx of calcium (Ca2+). In a feed-forward mechanism, K+ efflux causes further inflammasome activation, while Ca2+ influx promotes the release of extracellular vesicles (EVs). Pyroptotic vesicular shedding results in the release of membrane-bound inflammatory proteins, including cytokines, inflammasome components, and even fully formed inflammasome complexes. It is thought that the release of these EVs is a means to resolve the GSDMD pore formation through their budding off the membrane. If unresolved, the osmotic flux and cellular swelling can cause the cell to burst, resulting in the pro-inflammatory release of the cell’s intracellular contents into the extracellular environment and cellular death. Created with BioRender.com. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
