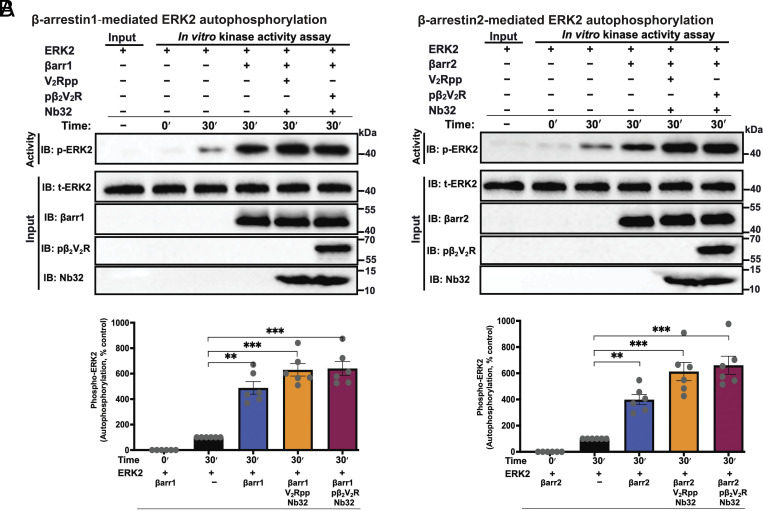
Fig. 2.
β-arrestin1/2 and their active states allosterically stimulate ERK2 autophosphorylation. (A and B, Top) shows representative western blots demonstrating that βarr1/2 and their active states (bound to V2Rpp or pβ2V2R) stimulate the autophosphorylation activity of ERK2. Reactions were performed in an endpoint format (30 min) at 30 °C using ERK2 (30 nM) with or without 1 μM βarr1 (A) or βarr2 (B) or each bound to V2Rpp or pβ2V2R together with Nb32. Samples were subsequently analyzed by western blotting with antibody specific for phospho-ERK1/2 (anti-ERK2-pT183/pY185). Total ERK2, βarr1/2, pβ2V2R, and Nb32 immunoblots represent individual input controls. Lower panels in A and B show bar graphs representing the extent of ERK2 autophosphorylation under the different conditions expressed as the average fold enhancement (means ± SEM) relative to a control reaction done with buffer (i.e., ERK2 alone treated as 100%). Data shown are means ± SEM (N = 6); significances by one-way ANOVA, followed by Dunnett's multiple comparison test. **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001.