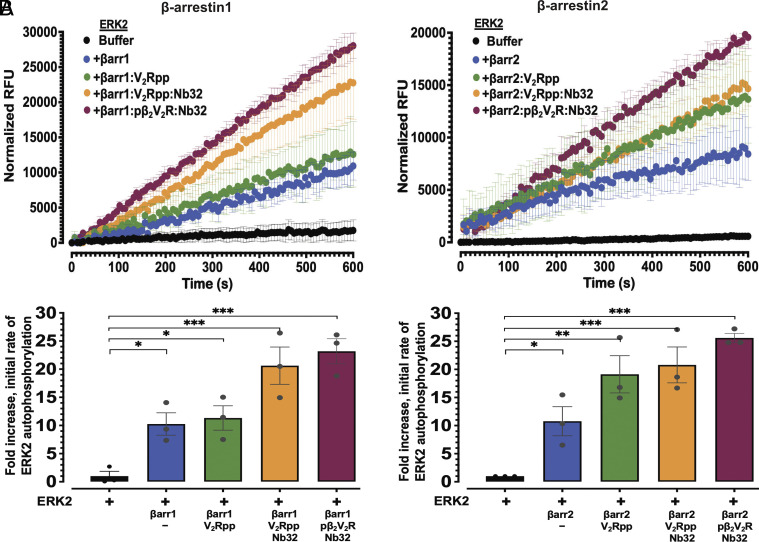
Fig. 3.
β-arrestin1/2 allosterically enhance the rate of ERK2 autophosphorylation. (A and B) show ERK2 autophosphorylation kinetics assessed using an in vitro real-time fluorescence-based kinase assay, in the absence or presence of basal state βarr1 (A) or βarr2 (B) or each in their active state forms bound to either V2Rpp or pβ2V2R together with Nb32. Kinase reactions were carried out with ERK2 (15 nM) and βarr1/2 (300 nM). Graphs (Top) represent the time course transitions of ERK2 autophosphorylation in relative fluorescence units (RFUs). Each experiment included control wells lacking ERK2 (with βarr 1 or 2, and ATP), and fluorescence signal data points from control reactions were subtracted to obtain corrected RFUs. (A and B, Lower) show quantification of ERK autophosphorylation presented as fold-enhancement of initial rates from each profile relative to vehicle control (ERK2 alone treated as onefold). Mean values are plotted, with error bars representing SEM (N = 3). Significances by one-way ANOVA, followed by Dunnett's multiple comparison test. *P ≤ 0.05; **P ≤ 0.01; and ***P ≤ 0.001.