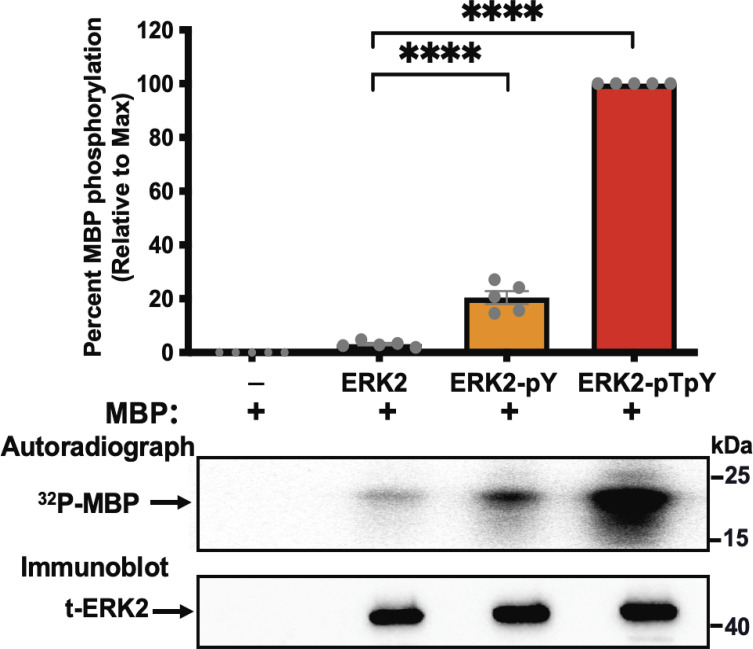
Fig. 5.
Monophosphorylation of ERK2 on Tyr185 substantially increases kinase activity. Bovine myelin basic protein (MBP) was incubated with ERK2 that was either unphosphorylated (ERK2), mono-phosphorylated ERK2 on Tyr185 (ERK2-pY), or dually phosphorylated on Thr183 and Tyr185 (ERK2-pTpY). Recombinant proteins were incubated at 37 °C for 1 h with [γ-32P] ATP as a phosphate source. Samples were separated on SDS-PAGE and 32P incorporation was assessed by autoradiography using a PhosphorImager. Total ERK2 immunoblot is shown as input control for each ERK2 species. Relative quantification of 32P incorporation is shown in the upper panel. Data are means ± SEM (N = 5). Statistical test: one-way ANOVA with Tukey's multiple comparison test, ****P ≤ 0.0001.