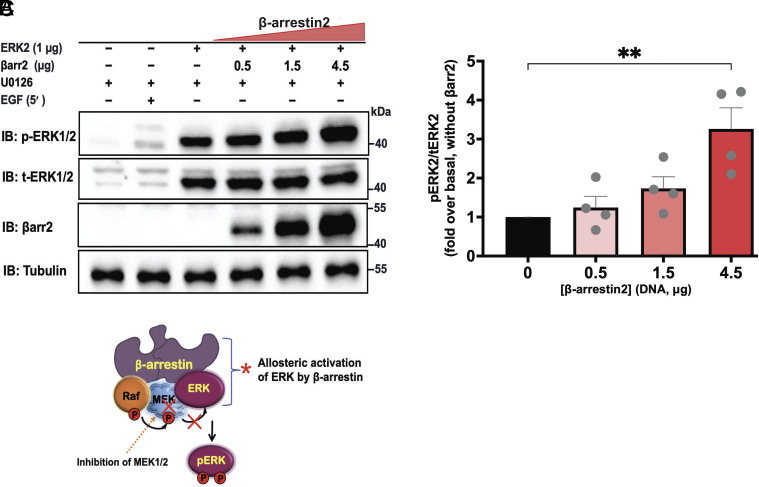
Fig. 7.
β-arrestin2 induces ERK2 activation in intact cells. (A) CRISPR/Cas9 βarr1/2 knockout HEK293 cells were transiently transfected with plasmids containing ERK2 and varying amounts of βarr2. After 48 h, cells were serum-starved and pretreated with vehicle (Dimethyl sulfoxide, DMSO) or 1 μM U0126 for 30 min. Lysates were immunoblotted for phospho-ERK2, total-ERK2, βarr2 (A1CT), and tubulin. (B) Quantification of MEK1/2- independent βarr-dependent ERK2 activation. Densitometry analysis of data from A is shown. Phospho-ERK signals were normalized to that of total-ERK2 and expressed as relative ratios to determine the fold response of βarr-mediated ERK2 activation compared to control. Data are means ± SEM of four independent experiments. Statistical test: one-way ANOVA with Bonferroni multiple comparison test, **P ≤ 0.01. (C) Cartoon showing a model of MEK-independent βarr mediated ERK Activation. The inhibition of MEK1/2 (chemically using U0126 herein) represents a tool to rule out the role of MEK1/2 in the allosteric activation of ERK1/2 by βarr1/2.