FIG. 4.
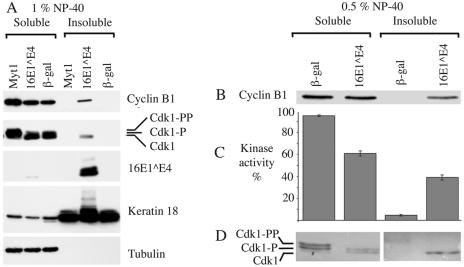
16E1∧E4 decreases the solubility of active Cdk1/cyclin B1. (A) G1/S-synchronized SiHa cells expressing Myt1, 16E1∧E4, or β-galactosidase were harvested at 20 h post-block release and fractionated with 1% NP-40 (NP-40-soluble fraction) and then with 1% SDS (NP-40-insoluble fraction). Fractions were Western blotted for 16E1∧E4, cyclin B1, Cdk1, keratin 18, and, as a control for fractionation, tubulin. (B) G1/S-synchronized SiHa cells expressing β-galactosidase or 16E1∧E4 were harvested at 20 h post-block release and fractionated first with 0.5% NP-40 (NP-40-soluble fraction) and then with 0.1% SDS (NP-40-insoluble fraction). The fractions were Western blotted for cyclin B1. (C) Cdk1/cyclin B1 was immunoprecipitated from fractions with anti-cyclin B1 monoclonal antibody. Immunoprecipitated complexes were used in an in vitro kinase assay to phosphorylate histone H1. 32P-labeled protein was detected with a phosphorimager and quantitated with ImageQuant. Kinase activity is expressed as a percentage of total activity in β-galactosidase- or 16E1∧E4-expressing cells. The results show the mean values of three experiments ± standard error of the mean. (D) Immunoprecipitated fractions were Western blotted for Cdk1.