Abstract
Background:
The prevalence of psoriasis is similar between men and women; however, evidence exists of sex- and gender-related differences in disease expression, impact, coping, and needs of patients with psoriasis. These differences are essential and should be considered in clinical practice and research.
Objective:
To compile available evidence on sex- and gender-related differences in psoriasis, identify the most critical gaps in clinical practice and research, and use it to propose strategies for improved clinical practice.
Methods:
Six European dermatologists selected the topics to consider according to their relevance in the dermatology setting with the support of methodologists. Evidence on sex- and gender-related differences was obtained by a scoping review based on search strategies in Medline and Cochrane Library from inception to October 2021 using the following terms: arthritis, psoriatic, psoriasis, gender, and sex. The panel discussed the results and proposed strategies by consensus.
Results:
The scoping review identified broad themes: (1) clinical expression, (2) severity and patient-reported outcomes, (3) psychosocial impact, (4) access to treatments and propensity to treat, (5) comorbidities, and (6) treatment effect. The strategies are based on these broad themes.
Limitations:
No risk of bias assessment was done due to the scoping nature of the review.
Conclusion:
This review offers insights into gender differences in psoriasis, providing a foundation for improving clinical practice and patient outcomes.
Keywords: gender, position statement, psoriasis, scoping review, sex
What is known about this subject in regard to women and their families?
Sex and gender are biological and social constructs, respectively, that modify the clinical expression, the impact, and, perhaps, the treatment of psoriasis.
What is new from this article as messages for women and their families?
We have identified that gender differences and unmet needs in women with psoriasis could easily be tackled by observing best practices already recommended.
Introduction
Sex, a biological construct, and gender, a social construct, are essential modifiers of chronic diseases at all levels, from awareness and diagnosis to access, treatment decisions, and outcomes.1,2 Adopting a gender-sensitive approach in any medical discipline or field involves 2 steps. First, to identify whether there are differences between men and women in terms of expression of the disease, access, or response to treatment, among others, and then to study in depth whether the existing differences could be reflecting actual biological differences or differences in roles, modes of coping, or even inequities led to by our perspective as doctors, frequently gender biased during education.3 The final and most needed step is to do something about the differences found, mainly if they are related to gender biases from the medical perspective.4
In psoriasis, there are differences between men and women in the epidemiology, severity, comorbidities, and treatment adherence that can be explained by several complex mechanisms, such as skin anatomy and physiological differences, hormonal, genetic, and epigenetic, as well as social, cultural, ethnic, and environmental factors.5 Such knowledge is crucial to improve the diagnosis, evaluation, and management of psoriatic patients and to support adopting a gender-sensitive approach in aspects in which this can be necessary.
Our objective was to compile the available evidence on gender-related differences in psoriasis, to identify gaps in clinical practice and research, and to propose strategies to adopt a gender-specific approach to improve the course of the disease and reduce possible gender inequities.
Methods
Evidence on sex- and gender-related differences was obtained by a scoping review based on search strategies in Medline and Cochrane Library from inception to October 2021 using synonyms of MeSH terms and free search terms of “psoriasis,” “gender,” and “sex” (search strategies and flow diagram are shown in Supplementary Table 1, http://links.lww.com/IJWD/A38 and Supplementary Figure 1, http://links.lww.com/IJWD/A36, respectively). Any type of design was eligible. Articles written in English or Spanish were eligible.
The compilation of themes, or charting, and their organization followed an inductive and deductive pattern. The inductive approach was guided by a meeting of the methodologists with the convenor (A.G.C.), who identified relevant topics, and the deductive approach was guided by reading the literature and identifying new themes. Two experienced reviewers outside the field of dermatology performed the scoping review. They synthesized the evidence in a report and presented it at a meeting, where it was discussed among the panel members from different European countries (Supplementary Figure 2, http://links.lww.com/IJWD/A37), after which these proposed strategies were approved by consensus. The evidence and discussions are presented herein as results.
Results
All the articles captured by the search strategies (n = 661) were downloaded, duplicates deleted, and the titles and abstracts screened for relation to the topic. After screening, we read the full text of 103 articles, of which 80 were retained for the synthesis (Supplementary Figure 1, http://links.lww.com/IJWD/A36).
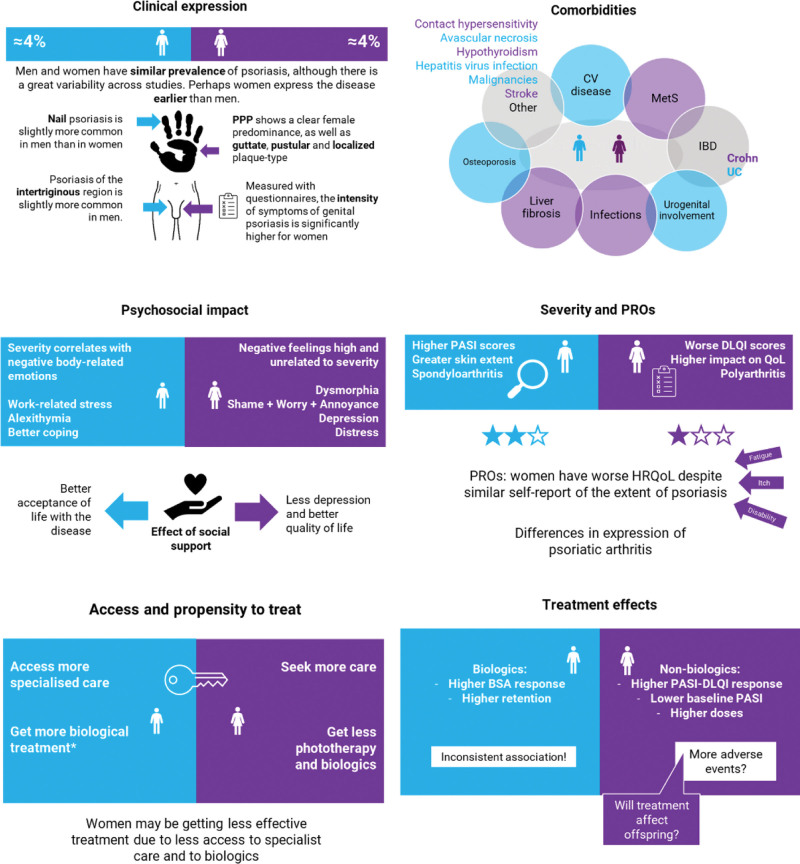
The scoping review identified the following themes: (1) clinical expression, (2) severity and patient-reported outcomes, (3) psychosocial impact, (4) access to treatments and propensity to treat, (5) comorbidities, and (6) treatment effects. Figure 1 is a succinct visual summary of the findings, later explained in the text. Results have been organized into themes and are each presented in the following structure: (1) evidence (knowledge), (2) gaps and research agenda, and (3) proposed strategies (compiled in Table 1).
Fig. 1.
A visual summary of the findings of the scoping review.
Table 1.
Proposed strategies to adopt a gender perspective in psoriasis
| Adopting a gender-sensitive approach in psoriasis implies | |
|---|---|
| 1 | Asking about symptoms and involvement of nonvisible areas and examining the whole body, not only the visible parts. |
| 2 | Including painful joint counts in regular examinations, including the evaluation of spine involvement, especially in men, as well as a measure of the impact of psoriasis on quality of life, especially in women. |
| 3 | Acquiring the skills to improve the identification of psychosocial problems in psoriatic patients and manage or refer them to adequate professionals for a tailored approach. |
| 4 | Choosing the systemic treatment after an informed shared decision with the patient. |
| 5 | Assessing comorbidity, regardless of the patient’s sex and gender identity, and agreeing with them on the appropriate treatment, including lifestyle changes. |
| 6 | Ideally, use shared decision-making aids with information on efficacy and side effects separated by sex. |
Clinical expression
Although it varies across studies, the overall estimated prevalence of psoriasis is similar between men and women, at around 4%.6,7 Some studies have detected differences between sexes in the location of psoriatic lesions. Nail psoriasis is slightly more common in men than in women.8–11 Contrarily, palmoplantar pustulosis shows a clear female predominance.9,10,12 Small studies show that guttate, pustular, and localized plaque-type lesions might be more common in women than in men.13 Genital involvement is reported more frequently in men than in women, especially in anogenital locations (59% in men vs 39% in women),14 and less intense a difference in the intertriginous region (19% in men vs 17% in women), practically equal.11
These differences in clinical expression need to be confirmed in descriptive studies of high quality, especially in terms of the representativeness of the patients (avoiding hospital samples and starting from a random sampling). Also, the pathophysiological explanations of these differences in the expression of psoriasis could open up interesting research hypotheses.15
Differences in the location of the lesions between men and women with psoriasis can have implications on the anamnesis and physical examination. In this sense, adopting a gender-sensitive approach implies asking about the symptoms and involvement of nonvisible areas and examining the whole body, not only the visible parts.
Measurement of severity and use of patient-reported outcomes
There are many definitions of the severity of psoriasis, including the extent of involvement or its impact on quality of life (QoL). In general, men show a greater area of the psoriatic lesions, with on average higher Psoriasis Area Severity Index (PASI) scores, while women tend to report a more significant impact on QoL, for example, worse Dermatology Life Quality Index (DLQI) scores.10,13,16–18
The higher impact on QoL in women is independent of age, self-reported extent, and severity measured by PASI or itch; this has been observed with different tools such as the DLQI, the QoL-Skinkex-17, and the short form-36.13,19–23 Women tend to present more frequently with pruritus than men (36% vs 25%) and of higher intensity.11,13,24 Also, the intensity of symptoms of genital psoriasis is significantly higher for women compared with men when measured with questionnaires despite the lesions being more frequent in men.25 In addition to a worse QoL, women with psoriasis report more frequent fatigue and health-related work disability than men.10
In the case of joint involvement, women with psoriasis also score worse in arthritis-related patient-reported outcomes, with more joints affected, generally polyarthritis, higher levels of pain, and poorer functional prognosis than men.18,26–30 In men, spondylarthritis is more frequent, being the type of physical impairment and activities affected differently between men and women.13,28,31 Differences by sex in the expression of psoriatic arthritis might be related to genetic background32 and could explain that in axial psoriatic arthritis, men develop more severe radiographic damage (odds ratio [OR] = 1.6) with higher restrictions of back movements and higher grades of sacroiliitis than women,26,27,33,34 while in peripheral psoriatic arthritis, the male gender is a predictor of remission and improved response, and the female gender is a predictor of worse functional scores and work disability.35–37 All these data indicate that women with polyarticular disease may need more aggressive treatment.30 Despite differences in the expression of joint involvement, the values of acute phase reactants or physician measures are similar in men and women.38,39
Many questions remain unanswered: Does the effect of treatments differ by sex on PASI and QoL measures? Is it gender- or sex-related variability? In the case of a gender gap, do interventions aimed at empowering women reduce the impact? In case of a sex-related gap, what factors lead to fatigue, itch, or other sensorial or emotional symptoms in both sexes?
Unfortunately, the results of randomized controlled trials are not systematically disaggregated by sex; therefore, these questions remain unanswered.
In addition to measuring the extent of skin involvement, a gender-sensitive assessment of severity must include measuring painful joint counts in regular examinations, including the evaluation of spine involvement, especially in men, and a measure of the impact of psoriasis on QoL, especially in women.
Psychosocial impact
Results from several studies have shown psychological differences by gender in psoriasis.40–46 Gender moderates the relationship between a patient’s subjective perceptions of the severity of the disease and emotional attitude towards the body. In men, disease severity seems to drive negative body-related emotions, while among women, these negative feelings towards the body appear to be high and unrelated to disease severity, probably due to the more rigid and pervasive appearance norms for women.42
Female patients are at higher risk of psychological distress than males, showing higher levels of stigmatization, social inhibition, negative affectivity, and neuroticism.43 The higher frequency of stigmatization in women, mainly motivated by the presence of skin lesions in visible areas, is an independent predictor of QoL impairment.47,48
In general, women feel more stress and worry than men, regardless of the extension of psoriasis,40 with significantly higher frequencies of shame (adjusted OR = 1.6), worry (adjusted OR = 1.8), and annoyance (adjusted OR = 1.9).45 The impact of the disease also shows differences by gender; usually, men show a more significant impact of work-related stress with fear of losing their job,10,49 whereas women show a worse perception of body image, a higher prevalence of body dysmorphic concerns,50 and impaired sexual dysfunction.51
Coping strategies and social support can buffer the negative effects of stress. Higher social support is associated with better acceptance of life with the disease in men and with lower depression and better QoL in women.41
The relationship between psoriasis and depression can be explained by different mechanisms, such as lower self-esteem, stigmatization and social withdrawal caused by skin lesions, and impaired QoL by systemic comorbidity. The association between female sex and depression in psoriasis has been shown in population-based52 and cross-sectional studies in which women showed less satisfaction with and acceptance of their body parts than the men, were more afraid of obesity and overweight,46,53 and experienced higher discomfort and negative impacts of psoriasis on mental health,54 with a higher probability of anxiety and depression.13,55 Despite the greater prevalence of depression in female patients, a review on suicidality in patients with psoriasis found no consistent results on the role of gender in this relationship.56
Occupational and psychosocial interventions are effective in coping effectively with stress and its various consequences on mental health.57 Basic skills for effective communication are a great start to psychoeducation and should be mandatory for all medical students and experienced doctors. Whether these skills should be designed explicitly for psoriasis patients is still being determined, but it would certainly not harm them. Some questionnaires can help us detect specific psychological problems; however, an honest, safe, and open conversation can also help us see patients needing further evaluation and professional psychological treatment.
Gender-sensitive dermatology would imply acquiring the skills to improve the identification of psychosocial problems in psoriatic patients and managing or referring them to adequate professionals for a tailored approach.
Access and treatment
Access to the healthcare system can substantially affect the prognosis of psoriasis. Delays in optimal care and undertreatment of the disease contribute to increased morbidity.28,30,58,59 Compared with men, female patients with psoriasis are more likely to seek care than men (adjusted OR = 1.47).60 Women often appear to be under-prescribed for ultraviolet treatment,61 though this question needs further study, and more men with psoriasis receive systemic treatments or biologics than women.16,39,62 However, this latter association is inconsistent across studies.17 Furthermore, despite achieving treatment targets, women tend to feel more disadvantaged in terms of life impact than men with psoriasis who achieve the same treatment target.63
Well-controlled studies should confirm these observations. We also do not know whether the gender of the physician influences treatment decisions in men and women with psoriasis, something that has been studied in arthritis, for instance. Professionals should be aware of this potential propensity to treat men more intensively than women. Some authors have pointed out that perhaps women are treated less frequently because they may have issues with the effect of treatments on pregnancy.64 This information needs to be understood in depth. In any case, an informed shared decision process should diminish inequities in treatment or its perception.
In a gender-sensitive approach, the specialist is accessible irrespective of sex. The choice of a systemic treatment is not based solely on skin involvement but on a shared decision with the patient after receiving information about the expected benefit of treatment options on the patient’s specific problems and their side effects.
Comorbidities
Inflammatory manifestations beyond the skin and the joints are frequent in psoriasis and affect different systems, with varying frequencies by gender.
Psoriasis is an independent risk factor for cardiovascular diseases, regardless of sex. Severe psoriasis is associated with a higher incidence of myocardial infarction in men (hazard ratio [HR] = 2.09) and women (HR = 3.23), and an increase in the risk of ischemic stroke, specifically in females (HR = 2.02).65 Population-based studies have shown that metabolic syndrome (MetS) and diabetes are more prevalent in women with psoriasis than without (37% vs 25% and 12% vs 9%), while in men, the association between MetS and psoriasis is negative.7 A higher prevalence of MetS in women has also been observed in case-control studies, with OR between 1.8966 and 3.19,67 although this association has not been consistent in other studies.68,69 On the other hand, the male sex has been associated with masked hypertension,10,70 body mass index ≥25, smoking, alcohol consumption, higher severity of psoriasis,13 risk of diabetes (HR = 1.57),71 and higher absolute 10-year cardiovascular risk, although in other studies women showed a higher risk for obesity (OR = 2.56), and systemic arterial hypertension (OR = 3.29) than men.69
Liver complications are relevant in patients with MetS and high alcohol intake, especially if prescribed drugs with hepatic metabolism, like methotrexate. To note, an Indian study (n = 134) found that MetS and female gender contributed more significantly to the development of liver fibrosis than methotrexate exposure.72
Other comorbidities with potentially different distribution between sexes in psoriasis are Crohn’s disease, higher in women than in men, while the opposite could be true for ulcerative colitis,73 nonpsoriatic urogenital inflammation, especially in men (OR = 3.47 with urethritis and prostatitis74), infections, especially in women with psoriatic arthritis (adjusted OR for male vs female = 0.4775), osteoporosis, more frequent in men,76 contact hypersensitivity, more frequent in women (27.7% vs 5.8%77), avascular necrosis (higher risk in men than in women, with adjusted HR = 2.2078), subclinical hypothyroidism (more frequent in women) and viral hepatitis (in men10).
Evidence on the effect of psoriasis on cardiovascular health underscores the need for sex-specific analyses in observational and real-world data studies.
In gender-sensitive dermatology, comorbidity assessment should be performed regardless of the patient’s sex and appropriate treatment, including lifestyle changes, should be agreed upon with the patient.
Treatment effects
In addition to methodological differences, the results about differences in rates of biological treatment between sexes may also be explained by gender differences in disease severity, treatment preferences, risk/benefit assessment by a physician, treatment access, and drug effectiveness.16,39,79
The analysis of psoriasis registries from Germany and Switzerland showed a higher PASI-DLQI response in women. However, most patients received nonbiologic agents, and women had lower PASI scores and less body weight at baseline, with relatively higher dosing of drugs.79 On the contrary, CORRONA registry results showed that women are less likely to have a body surface area response to anti-TNFs (adjusted OR = 0.53).80 However, the results should be cautiously interpreted due to residual confounding. A post hoc analysis of phase 3 trials and long-term extension studies did not detect differences in the response to tofacitinib compared with placebo between men and women, despite the response behavior being different between men and women if the treatment group was not considered.81
Female sex is a predictor of biologic discontinuation in drug-survival analysis.82,83 Results of a meta-analysis on the predictors of biologic persistence in psoriasis showed that female patients are more likely to discontinue therapy (HR = 1.22) and discontinue due to adverse events (HR = 2.16).84 The reason for worse drug persistence rates in women is unknown. However, it is hypothesized that it may be due to biological differences in developing antidrug antibodies and therapeutic dissatisfaction.84,85 Other authors have found no differences in suspension due to remission or ineffectiveness between males and females.17 Discrepancies between these results may be due to several factors. First, drug survival does not only incorporate drug effectiveness but also safety, reimbursement, availability of alternative treatment options, and expectations of physicians and patients, and all these factors may explain the inconsistency between PASI or body surface area response rates and drug retention rates.86,87 Second, methodological differences, residual confounding, and selection biases may also contribute.17
Regarding unwanted effects, women generally experience adverse drug reactions more frequently than men.17,84,88 The underlying mechanisms are not entirely clear, but sex differences in pharmacokinetics strongly predict sex-specific toxicities for women.89
In summary, the data about the influence of gender in response to biological therapy are inconsistent and require further exploration. Pharmaceutical companies and regulatory agencies should pay more attention to pharmacokinetic data to find gender-appropriate dosing and improve the safety profile of women. Gender-specific analyses are needed in clinical trials and world-real studies to better understand the gender influences on drug responses.
In an ideal world and with a gender-sensitive perspective, shared decision-making aids include information on treatment responses to specific symptoms and side effects by sex.
Discussion
We aimed to explore what topics under the umbrella of sex differences and gender perspectives had already been studied and call the attention of dermatologists to what should be a gender-sensitive approach in clinical practice. We did not want to review the literature merely.15 As a general note of caution, this review did not intend to measure or critically evaluate the studies. A scoping review is meant to identify topics in the literature and cannot properly support recommendations.90 We chose this methodology to avoid being guided by our previous knowledge or prejudices. It can serve as a structure for appropriate systematic reviews and recommendations.
By the time we were publishing this review, an excellent review by Guillet et al.15 was published in this journal. Interestingly, both reviews are complementary, as Guillet et al.15 present a more detailed description of specific treatments and pregnancy and fertility issues, and ours stresses the differences in the detection of comorbidities, especially arthritis, and psychosocial impact, that is, try to focus on gender rather than sex. In their review, Guillet et al.15 did not find major differences in the response to treatments between men and women. The absence of differences between sexes in clinical trials could be explained by a limited sample size to detect slight differences, or an unbalanced distribution of baseline variables between sexes (higher PASI in men, higher depression in women, higher disability in women, etc.). A study published after our review in 2023 combining information from 3 large trials of tofacitinib found no difference in the effect compared with placebo between men and women, but they did not adjust for baseline differences.81
Gender roles are a moving target and will be until one day they disappear. We all, men and women, adopt different perspectives and roles depending on our origin, culture, and level of education and empowerment, but also time, context, and activity. Nowadays, gender is a variable that is challenging to measure. Meanwhile, we have access to research that mixes both constructs, sex (biological) and gender (social), or what is worse, no research at all, despite, inevitably, the biology being different.
Knowledge of the differences may prompt pathogenic hypotheses, recognize other forms of the disease, and acknowledge and correct inequities. As an example, understanding the impact of gender on drug effects may help to individualize biological therapy and improve outcomes and satisfaction with the treatment, thus being a step towards truly individualized medicine. Also, a better understanding of the psychological profile—culturally different between genders—allows for targeted approaches toward ameliorating the psychosocial disturbances associated with psoriasis. This acknowledgment would help prevent depressive symptoms in women with psoriasis—for example, improving body image or reducing negative emotions about one’s appearance—and enhance the mood of alexithymic men. Furthermore, the protection of women’s health highlighted by our review is essential for advocating gender equality and sustainability, which are recommended by the United Nations Women.91
This review has limitations, as its scoping nature, without proper evaluation and quantification of the results, cannot be used to support specific actions. We recommend developing evidence-based recommendations using specific questions on the themes the scoping review elicited. Our proposed strategies can be used to frame the items to include in such a document.
The strategies herein proposed have the broad intention of improving dermatological practice. Although they might not seem specific for any gender, they benefit women by positioning pruritus, psychological aspects, and rare psoriasis locations at the skin extension level when making decisions. After all, when one shapes practices favoring less privileged or vulnerable people (eg, people with disabilities, mental burdens, or financial constraints), the changes implemented benefit all. Therefore, adopting a gender-sensitive approach would benefit not only women but also men.
Conclusion
This evidence and proposed strategies emphasize the need to consider sex- and gender-related factors as valuable qualifiers of systemic therapy decision-making in routine practice care and motivate a gender perspective in managing psoriasis.
Conflicts of interest
A.G.C. has served as a consultant for Abbie, Janssen, Novartis, Almirall, Celgene, and Leo Pharma, receiving grants/other payments. M.M.C. has received honoraria for participation on advisory boards, as a speaker and/or for consultancy, from AbbVie, Almirall, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sandoz, Servier, Sun Pharma, UCB Pharma. A.D. has received honoraria for participation on advisory boards, as a speaker and/or for consultancy, from AbbVie, Celgene, Eli Lilly, Janssen, Novartis, UCB Pharma, Leo Pharma, and Medac Pharma. T.H. has received consultancy, speaker fees, and/or research funding from AbbVie, Almirall, Amgen, Biogen, Bristol Myers Squibb, Celgene, Janssen, Leo Pharma, Eli Lilly, Novartis, Pfizer Inc, Roche, Sandoz, Sanofi, UCB Pharma. N.M. has received Honoraria for participation on advisory boards, as a speaker, and/or for a consultancy from AbbVie, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, and UCB Pharma. There is no conflict of interest for the remaining author.
Funding
This project was funded by an unrestricted grant from UCB Biopharma SRL. The content has been developed independently from UCB. Opinions or statements in this publication do not represent a UCB opinion.
Study approval
Scoping reviews are not subject to ethics approval or disapproval.
Author contributions
AGC: Coordinated the work. AGC, MMC, AD, TH, EK, and NM participated in making the research questions, interpreting the results of the review, proposing the strategies, and writing the paper.
Acknowledgments
We thank Maria Jesús García de Yébenes and Loreto Carmona from Inmusc for their methodological help and guidance.
Supplementary data
Supplementary material associated with this article can be found at http://links.lww.com/IJWD/A38, http://links.lww.com/IJWD/A36, and http://links.lww.com/IJWD/A37.
Supplementary Material
Footnotes
Published online 1 November 2023
References
- 1.Denton M, Prus S, Walters V. Gender differences in health: a Canadian study of the psychosocial, structural and behavioural determinants of health. Soc Sci Med 2004;58:2585–600. doi: 10.1016/j.socscimed.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 2020;396:565–82. doi: 10.1016/s0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lent B, Bishop JE. Sense and sensitivity: developing a gender issues perspective in medical education. J Womens Health 1998;7:339–42. doi: 10.1089/jwh.1998.7.339. [DOI] [PubMed] [Google Scholar]
- 4.Risberg G, Johansson EE, Hamberg K. A theoretical model for analysing gender bias in medicine. Int J Equity Health 2009;8:28. doi: 10.1186/1475-9276-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi SS, Ramot Y. Gender differences in psoriasis. In: Tur E, Maibach H, eds. Gender and Dermatology. Springer, Cham; 2018:63–81. doi: 10.1007/978-3-319-72156-9_7. [Google Scholar]
- 6.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377–85. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 7.Sondermann W, Djeudeu Deudjui DA, Korber A, et al. Psoriasis, cardiovascular risk factors and metabolic disorders: sex-specific findings of a population-based study. J Eur Acad Dermatol Venereol 2020;34:779–86. doi: 10.1111/jdv.16029. [DOI] [PubMed] [Google Scholar]
- 8.Augustin M, Reich K, Blome C, Schäfer I, Laass A, Radtke MA. Nail psoriasis in Germany: epidemiology and burden of disease. Br J Dermatol 2010;163:580–5. doi: 10.1111/j.1365-2133.2010.09831.x. [DOI] [PubMed] [Google Scholar]
- 9.Benzian-Olsson N, Dand N, Chaloner C, et al. ; ERASPEN Consortium and the APRICOT and PLUM Study Team. Association of clinical and demographic factors with the severity of palmoplantar pustulosis. JAMA Dermatol 2020;156:1216–22. doi: 10.1001/jamadermatol.2020.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo D, Cassano N, Bellia G, Vena G. Gender medicine and psoriasis. World J Dermatology 2014;3:36–44. doi: 10.5314/wjd.v3.i3.36. [Google Scholar]
- 11.Murer C, Sgier D, Mettler SK, et al. Gender differences in psoriasis: a Swiss online psoriasis survey. Arch Dermatol Res 2021;313:89–94. doi: 10.1007/s00403-020-02066-1. [DOI] [PubMed] [Google Scholar]
- 12.Brunasso AM, Puntoni M, Aberer W, Delfino C, Fancelli L, Massone C. Clinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series study. Br J Dermatol 2013;168:1243–51. doi: 10.1111/bjd.12223. [DOI] [PubMed] [Google Scholar]
- 13.Napolitano M, Mastroeni S, Fania L, et al. Sex- and gender-associated clinical and psychosocial characteristics of patients with psoriasis. Clin Exp Dermatol 2020;45:705–11. doi: 10.1111/ced.14218. [DOI] [PubMed] [Google Scholar]
- 14.Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol 2015;72:978–83. doi: 10.1016/j.jaad.2015.02.1127. [DOI] [PubMed] [Google Scholar]
- 15.Guillet C, Seeli C, Nina M, Maul LV, Maul JT. The impact of gender and sex in psoriasis: what to be aware of when treating women with psoriasis. Int J Womens Dermatol 2022;8:e010. doi: 10.1097/jw9.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hägg D, Sundström A, Eriksson M, Schmitt-Egenolf M. Severity of psoriasis differs between men and women: a study of the clinical outcome measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish register patients. Am J Clin Dermatol 2017;18:583–90. doi: 10.1007/s40257-017-0274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Fernández CP, Carretero G, Rivera R, et al. ; the BIOBADADERM Study Group. Effect of sex in systemic Psoriasis therapy: differences in prescription, effectiveness and safety in the BIOBADADERM prospective cohort. Acta Derm Venereol 2021;101:adv00354. doi: 10.2340/00015555-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nas K, Capkin E, Dagli AZ, et al. ; Anatolian Group for the Assessment in Rheumatic Diseases (ANGARD). Gender specific differences in patients with psoriatic arthritis. Mod Rheumatol 2017;27:345–9. doi: 10.1080/14397595.2016.1193105. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Torres RM, Pita-Fernández S, Fonseca E. Quality of life and related factors in a cohort of plaque-type psoriasis patients in La Coruña, Spain. Int J Dermatol 2014;53:e507–11. doi: 10.1111/ijd.12294. [DOI] [PubMed] [Google Scholar]
- 20.Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol 2004;51:704–8. doi: 10.1016/j.jaad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Mabuchi T, Yamaoka H, Kojima T, Ikoma N, Akasaka E, Ozawa A. Psoriasis affects patient’s quality of life more seriously in female than in male in Japan. Tokai J Exp Clin Med 2012;37:84–8. [PubMed] [Google Scholar]
- 22.Youn SW, Lee JH, Yu DY, et al. The relationship between clinical characteristics including presence of exposed lesions and health-related quality of life (HRQoL) in patients with psoriasis: analysis from the nationwide epidemiologic study for psoriasis in Korea (EPI-PSODE study). J Eur Acad Dermatol Venereol 2018;32:1499–506. doi: 10.1111/jdv.14865. [DOI] [PubMed] [Google Scholar]
- 23.Ng CY, Yang YW, Liu SH, et al. SF-36 healthy survey on psoriasis quality-of-life: a study of 414 Taiwanese patients. J Dermatol 2015;42:159–65. doi: 10.1111/1346-8138.12748. [DOI] [PubMed] [Google Scholar]
- 24.Damiani G, Cazzaniga S, Conic RR, Naldi L, Psocare Registry N. Pruritus characteristics in a large Italian cohort of psoriatic patients. J Eur Acad Dermatol Venereol 2019;33:1316–24. doi: 10.1111/jdv.15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeuwis KA, van de Kerkhof PC, Massuger LF, de Hullu JA, van Rossum MM. Patients’ experience of psoriasis in the genital area. Dermatology 2012;224:271–6. doi: 10.1159/000338858. [DOI] [PubMed] [Google Scholar]
- 26.Eder L, Thavaneswaran A, Chandran V, Gladman DD. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann Rheum Dis 2013;72:578–82. doi: 10.1136/annrheumdis-2012-201357. [DOI] [PubMed] [Google Scholar]
- 27.Landi M, Maldonado-Ficco H, Perez-Alamino R, et al. ; RESPONDIA Group. Fundación Reumatológica Argentina “Dr. Osvaldo García Morteo. Gender differences among patients with primary ankylosing spondylitis and spondylitis associated with psoriasis and inflammatory bowel disease in an iberoamerican spondyloarthritis cohort. Medicine (Baltim) 2016;95:e5652. doi: 10.1097/md.0000000000005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nas K, Kilic E, Tekeoglu I, et al. The effect of gender on disease activity and clinical characteristics in patients with axial psoriatic arthritis. Mod Rheumatol 2021;31:869–74. doi: 10.1080/14397595.2020.1812870. [DOI] [PubMed] [Google Scholar]
- 29.Ros Expósito S, Rodríguez Moreno J, Gómez Vaquero C, Campoy Reolid E, Roig Escofet D. Prognostic factors in the evolution of psoriatic arthritis. Med Clin (Barc) 1997;108:133–5. [PubMed] [Google Scholar]
- 30.Theander E, Husmark T, Alenius GM, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann Rheum Dis 2014;73:407–13. doi: 10.1136/annrheumdis-2012-201972. [DOI] [PubMed] [Google Scholar]
- 31.Queiro R, Sarasqueta C, Torre JC, Tinturé T, López-Lagunas I. Comparative analysis of psoriatic spondyloarthropathy between men and women. Rheumatol Int 2001;21:66–8. doi: 10.1007/s002960100135. [DOI] [PubMed] [Google Scholar]
- 32.Queiro R, Tejon P, Coto P, et al. Could the clinical differences between men and women with psoriatic arthritis be explained in part by genetic factors? Clin Exp Rheumatol 2013;31:324–5. [PubMed] [Google Scholar]
- 33.Geijer M, Lindqvist U, Husmark T, et al. The Swedish early psoriatic arthritis registry 5-year followup: substantial radiographic progression mainly in men with high disease activity and development of dactylitis. J Rheumatol 2015;42:2110–7. doi: 10.3899/jrheum.150165. [DOI] [PubMed] [Google Scholar]
- 34.Gladman DD, Brubacher B, Buskila D, Langevitz P, Farewell VT. Psoriatic spondyloarthropathy in men and women: a clinical, radiographic, and HLA study. Clin Invest Med 1992;15:371–5. [PubMed] [Google Scholar]
- 35.Eder L, Gladman DD. Predictors for clinical outcome in psoriatic arthritis - what have we learned from cohort studies? Expert Rev Clin Immunol 2014;10:763–70. doi: 10.1586/1744666x.2014.905741. [DOI] [PubMed] [Google Scholar]
- 36.Queiro R, Tejón P, Coto P, et al. Clinical differences between men and women with psoriatic arthritis: relevance of the analysis of genes and polymorphisms in the major histocompatibility complex region and of the age at onset of psoriasis. Clin Dev Immunol 2013;2013:482691. doi: 10.1155/2013/482691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallenius M, Skomsvoll JF, Koldingsnes W, et al. Work disability and health-related quality of life in males and females with psoriatic arthritis. Ann Rheum Dis 2009;68:685–9. doi: 10.1136/ard.2008.092049. [DOI] [PubMed] [Google Scholar]
- 38.Kenar G, Yarkan H, Zengin B, Can G, Birlik M, Önen F. Gender does not make a difference in “composite psoriatic disease activity index (CPDAI)” in patients with psoriatic arthritis. Rheumatol Int 2018;38:2069–76. doi: 10.1007/s00296-018-4153-7. [DOI] [PubMed] [Google Scholar]
- 39.Lesuis N, Befrits R, Nyberg F, van Vollenhoven RF. Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Med 2012;10:82. doi: 10.1186/1741-7015-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finzi A, Colombo D, Caputo A, et al. ; PSYCHAE Study Group. Psychological distress and coping strategies in patients with psoriasis: the PSYCHAE Study. J Eur Acad Dermatol Venereol 2007;21:1161–9. doi: 10.1111/j.1468-3083.2007.02079.x. [DOI] [PubMed] [Google Scholar]
- 41.Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res 2012;304:421–32. doi: 10.1007/s00403-012-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakuta P, Przybyla-Basista H. Toward a better understanding of social anxiety and depression in psoriasis patients: the role of determinants, mediators, and moderators. J Psychosom Res 2017;94:32–8. doi: 10.1016/j.jpsychores.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Lim DS, Bewley A, Oon HH. Psychological profile of patients with psoriasis. Ann Acad Med Singap 2018;47:516–22. [PubMed] [Google Scholar]
- 44.Sampogna F, Chren MM, Melchi CF, Pasquini P, Tabolli S, Abeni D; Italian Multipurpose Psoriasis Research on Vital Experiences (Improve) Study Group. Age, gender, quality of life and psychological distress in patients hospitalized with psoriasis. Br J Dermatol 2006;154:325–31. doi: 10.1111/j.1365-2133.2005.06909.x. [DOI] [PubMed] [Google Scholar]
- 45.Sampogna F, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences (IMPROVE) investigators. Living with psoriasis: prevalence of shame, anger, worry, and problems in daily activities and social life. Acta Derm Venereol 2012;92:299–303. doi: 10.2340/00015555-1273. [DOI] [PubMed] [Google Scholar]
- 46.Wojtyna E, Lakuta P, Marcinkiewicz K, Bergler-Czop B, Brzezinska-Wcislo L. Gender, body image and social support: biopsychosocial deter-minants of depression among patients with psoriasis. Acta Derm Venereol 2017;97:91–7. doi: 10.2340/00015555-2483. [DOI] [PubMed] [Google Scholar]
- 47.Hawro M, Maurer M, Weller K, et al. Lesions on the back of hands and female gender predispose to stigmatization in patients with psoriasis. J Am Acad Dermatol 2017;76:648–54.e2. doi: 10.1016/j.jaad.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 48.Schmid-Ott G, Künsebeck HW, Jäger B, et al. Significance of the stigmatization experience of psoriasis patients: a 1-year follow-up of the illness and its psychosocial consequences in men and women. Acta Derm Venereol 2005;85:27–32. doi: 10.1080/000155550410021583. [DOI] [PubMed] [Google Scholar]
- 49.Gupta MA, Gupta AK. Age and gender differences in the impact of psoriasis on quality of life. Int J Dermatol 1995;34:700–3. doi: 10.1111/j.1365-4362.1995.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 50.da Silva N, Augustin M, Langenbruch A, et al. Sex-related impairment and patient needs/benefits in anogenital psoriasis: difficult-to-communicate topics and their impact on patient-centred care. PLoS One 2020;15:e0235091. doi: 10.1371/journal.pone.0235091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molina-Leyva A, Jiménez-Moleón JJ, Naranjo-Sintes R, Ruiz-Carrascosa JC. Sexual dysfunction in psoriasis: a systematic review. J Eur Acad Dermatol Venereol 2015;29:649–55. doi: 10.1111/jdv.12845. [DOI] [PubMed] [Google Scholar]
- 52.Hu SC, Chen GS, Tu HP. Epidemiology of depression in patients with psoriasis: a nationwide population-based cross-sectional study. Acta Derm Venereol 2019;99:530–8. doi:10.2340/00015555-3145. [DOI] [PubMed] [Google Scholar]
- 53.Rosinska M, Rzepa T, Szramka-Pawlak B, Zaba R. Body image and depressive symptoms in person suffering from psoriasis. Psychiatr Pol 2017;51:1145–52. doi: 10.12740/PP/68948. [DOI] [PubMed] [Google Scholar]
- 54.Böhm D, Stock Gissendanner S, Bangemann K, et al. Perceived relationships between severity of psoriasis symptoms, gender, stigmatization and quality of life. J Eur Acad Dermatol Venereol 2013;27:220–6. doi: 10.1111/j.1468-3083.2012.04451.x. [DOI] [PubMed] [Google Scholar]
- 55.Kouris A, Christodoulou C, Stefanaki C, et al. Quality of life and psychosocial aspects in Greek patients with psoriasis: a cross-sectional study. An Bras Dermatol 2015;90:841–5. doi: 10.1590/abd1806-4841.20154147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu KK, Armstrong AW. Suicidality among psoriasis patients: a critical evidence synthesis. G Ital Dermatol Venereol 2019;154:56–63. doi: 10.23736/S0392-0488.18.06112-6. [DOI] [PubMed] [Google Scholar]
- 57.Maydych V. The interplay between stress, inflammation, and emotional attention: relevance for depression. Front Neurosci 2019;13:384. doi: 10.3389/fnins.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eder L, Harvey P, Chandran V, et al. Gaps in diagnosis and treatment of cardiovascular risk factors in patients with psoriatic disease: an international multicenter study. J Rheumatol 2018;45:378–84. doi: 10.3899/jrheum.170379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rusman T, van Vollenhoven RF, van der Horst-Bruinsma IE. Gender differences in axial spondyloarthritis: women are not so lucky. Curr Rheumatol Rep 2018;20:35. doi: 10.1007/s11926-018-0744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhutani T, Wong JW, Bebo BF, Armstrong AW. Access to health care in patients with psoriasis and psoriatic arthritis: data from National Psoriasis Foundation survey panels. JAMA Dermatol 2013;149:717–21. doi: 10.1001/jamadermatol.2013.133. [DOI] [PubMed] [Google Scholar]
- 61.Waernulf L, Moberg C, Henriksson EW, Evengard B, Nyberg F. Patients’ views on care and treatment after phototherapy for psoriasis and atopic eczema including a gender perspective. J Dermatolog Treat 2008;19:233–40. doi: 10.1080/09546630801955127. [DOI] [PubMed] [Google Scholar]
- 62.White D, O’Shea SJ, Rogers S. Do men have more severe psoriasis than women? J Eur Acad Dermatol Venereol 2012;26:126–7. doi: 10.1111/j.1468-3083.2011.04026.x. [DOI] [PubMed] [Google Scholar]
- 63.Orbai AM, Perin J, Gorlier C, et al. Determinants of patient-reported psoriatic arthritis impact of disease: an analysis of the association with sex in 458 patients from fourteen countries. Arthritis Care Res (Hoboken) 2020;72:1772–9. doi: 10.1002/acr.24090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brophy S, Taylor G, Blake D, Calin A. The interrelationship between sex, susceptibility factors, and outcome in ankylosing spondylitis and its associated disorders including inflammatory bowel disease, psoriasis, and iritis. J Rheumatol 2003;30:2054–8. [PubMed] [Google Scholar]
- 65.Jung KJ, Kim TG, Lee JW, et al. Increased risk of atherosclerotic cardiovascular disease among patients with psoriasis in Korea: A 15-year nationwide population-based cohort study. J Dermatol 2019;46:859–66. doi: 10.1111/1346-8138.15052. [DOI] [PubMed] [Google Scholar]
- 66.Mebazaa A, El Asmi M, Zidi W, et al. Metabolic syndrome in Tunisian psoriatic patients: prevalence and determinants. J Eur Acad Dermatol Venereol 2011;25:705–9. doi: 10.1111/j.1468-3083.2010.03856.x. [DOI] [PubMed] [Google Scholar]
- 67.Zindanci I, Albayrak O, Kavala M, et al. Prevalence of metabolic syndrome in patients with psoriasis. ScientificWorldJournal 2012;2012:312463. doi: 10.1100/2012/312463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meziane M, Kelati A, Najdi A, Berraho A, Nejjari C, Mernissi FZ. Metabolic syndrome in Moroccan patients with psoriasis. Int J Dermatol 2016;55:396–400. doi: 10.1111/ijd.12623. [DOI] [PubMed] [Google Scholar]
- 69.Paschoal RS, Silva DA, Cardili RN, Souza CDS. Metabolic syndrome, C-reactive protein and cardiovascular risk in psoriasis patients: a cross-sectional study. An Bras Dermatol 2018;93:222–8. doi: 10.1590/abd1806-4841.20186397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adisen E, Uzun S, Erduran F, Gurer MA. Prevalence of smoking, alcohol consumption and metabolic syndrome in patients with psoriasis. An Bras Dermatol 2018;93:205–11. doi: 10.1590/abd1806-4841.20186168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milan R, LeLorier J, Litvinov IV, Dasgupta K, Rahme E. Sex differences in the risk of diabetes mellitus among individuals with psoriasis: a retrospective cohort study in Québec, Canada. J Am Acad Dermatol 2021;85:213–5. doi: 10.1016/j.jaad.2020.07.082. [DOI] [PubMed] [Google Scholar]
- 72.Mahajan R, Dogra S, Handa S, et al. Metabolic syndrome and female gender, but not methotrexate, are the important associations of significant liver fibrosis in patients with moderate-to-severe psoriasis as detected by transient elastography. Indian J Dermatol Venereol Leprol 2020;86:649–55. doi: 10.4103/ijdvl.IJDVL_152_19. [DOI] [PubMed] [Google Scholar]
- 73.Egeberg A, Thyssen JP, Burisch J, Colombel JF. Incidence and risk of inflammatory bowel disease in patients with psoriasis-a nationwide 20-year cohort study. J Invest Dermatol 2019;139:316–23. doi: 10.1016/j.jid.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 74.Peluso R, Iervolino S, Vitiello M, Bruner V, Lupoli G, Di Minno MN. Extra-articular manifestations in psoriatic arthritis patients. Clin Rheumatol 2015;34:745–53. doi: 10.1007/s10067-014-2652-9. [DOI] [PubMed] [Google Scholar]
- 75.Haddad A, Li S, Thavaneswaran A, Cook RJ, Chandran V, Gladman DD. The incidence and predictors of infection in psoriasis and psoriatic arthritis: results from longitudinal observational cohorts. J Rheumatol 2016;43:362–6. doi: 10.3899/jrheum.140067. [DOI] [PubMed] [Google Scholar]
- 76.Dreiher J, Weitzman D, Cohen AD. Psoriasis and osteoporosis: a sex-specific association? J Invest Dermatol 2009;129:1643–9. doi: 10.1038/jid.2008.432. [DOI] [PubMed] [Google Scholar]
- 77.Jovanović M, Boza P, Karadaglić D, et al. Contact sensitivity in patients with psoriasis in Vojvodina. Int Arch Allergy Immunol 2009;148:311–20. doi: 10.1159/000170385. [DOI] [PubMed] [Google Scholar]
- 78.Chiu HY, Wang IT, Huang WF, Tsai YW, Shiu MN, Tsai TF. Increased risk of avascular necrosis in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol 2017;76:903–10.e1. doi: 10.1016/j.jaad.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Maul JT, Augustin M, Sorbe C, et al. Association of sex and systemic therapy treatment outcomes in psoriasis: a two-country, multicentre, prospective, noninterventional registry study. Br J Dermatol 2021;185:1160–8. doi: 10.1111/bjd.20387. [DOI] [PubMed] [Google Scholar]
- 80.Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to anti-tumor necrosis factor therapies in patients with moderate to severe psoriasis: real-world data from the US Corrona Psoriasis Registry. J Dermatolog Treat 2021;32:302–9. doi: 10.1080/09546634.2019.1656797. [DOI] [PubMed] [Google Scholar]
- 81.Lihi E, Dafna DG, Philip M, et al. Sex differences in the efficacy, safety and persistence of patients with psoriatic arthritis treated with tofacitinib: a post-hoc analysis of phase 3 trials and long-term extension. RMD Open 2023;9:e002718. doi: 10.1136/rmdopen-2022-002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Graier T, Weger W, Sator PG, et al. Effectiveness and clinical predictors of drug survival in psoriasis patients receiving apremilast: a registry analysis. JAAD Int 2021;2:62–75. doi: 10.1016/j.jdin.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zweegers J, van den Reek JM, van de Kerkhof PC, et al. Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side-effects in patients with psoriasis treated with adalimumab, etanercept or ustekinumab in daily practice: a prospective, comparative, long-term drug-survival study from the BioCAPTURE registry. Br J Dermatol 2016;175:340–7. doi: 10.1111/bjd.14552. [DOI] [PubMed] [Google Scholar]
- 84.Mourad A, Straube S, Armijo-Olivo S, Gniadecki R. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol 2019;181:450–8. doi: 10.1111/bjd.17738. [DOI] [PubMed] [Google Scholar]
- 85.van der Schoot LS, van den Reek J, Groenewoud JMM, et al. Female patients are less satisfied with biological treatment for psoriasis and experience more side-effects than male patients: results from the prospective BioCAPTURE registry. J Eur Acad Dermatol Venereol 2019;33:1913–20. doi: 10.1111/jdv.15733. [DOI] [PubMed] [Google Scholar]
- 86.Graier T, Salmhofer W, Jonak C, et al. Biologic drug survival rates in the era of anti-interleukin-17 antibodies: a time-period-adjusted registry analysis. Br J Dermatol 2021;184:1094–105. doi: 10.1111/bjd.19701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wolf P. Systemic antipsoriatic treatment: do women respond better than men and if so, why? Br J Dermatol 2021;185:1088–9. doi: 10.1111/bjd.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colombo D, Banfi G, Cassano N, et al. ; GENDER ATTENTION study group. The GENDER ATTENTION observational study: gender and hormonal status differences in the incidence of adverse events during cyclosporine treatment in psoriatic patients. Adv Ther 2017;34:1349–63. doi: 10.1007/s12325-017-0526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zucker I, Prendergast BJ. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol Sex Differ. 2020;11:32. doi: 10.1186/s13293-020-00308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tait CA, Abdillahi I, Wong W, Smith-Cannoy H, Siddiqi A. Can the health effects of widely-held societal norms be evaluated? An analysis of the United Nations convention on the elimination of all forms of discrimination against women (UN-CEDAW). BMC Public Health. 2019;19:279. doi: 10.1186/s12889-019-6607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.