Abstract
Heart failure is an increasing public health issue with substantial morbidity and mortality rates. This study aimed to evaluate the efficacy, safety, and long-term outcomes of angiotensin receptor neprilysin inhibitor (ARNi) in the treatment of heart failure with reduced ejection fraction (HFrEF) 5 years after treatment initiation. This retrospective study analyzed a cohort of 75 patients diagnosed with HFrEF over a period of 5 years after the initiation of ARNi therapy. The initial clinical condition, laboratory and echocardiographic measurements including left ventricular ejection fraction (LVEF), New York Heart Association functional classes (NYHA-FC) and the prognostic nutritional index were compared to the corresponding values obtained after a 5-year period of ARNi therapy. In addition, the number of annual hospitalizations, mortality rates and any history of adverse effects during the follow-up period were recorded. The N-terminal pro-brain natriuretic peptide (NT-proBNP) level, LVEF, and NYHA-FC values demonstrated significant improvement at the end of the 5-year follow-up period (all parameters, P < .001). Although the observed increase in the prognostic nutritional index was not statistically significant (P = .077), it is worth noting. A significant reduction in daily diuretic doses and hospitalizations due to heart failure was observed following the use of ARNi (all comparisons, P < .001). The prevalence of hypotension was around 16% (being symptomatic in 4%), making it the most frequently observed adverse event. The 5-year cardiovascular mortality rate was 17.3%. The use of ARNi in HFrEF patients was associated with a notable improvement in NYHA-FC, LVEF, and NT-proBNP levels in the long-term, while also leading to a better nutritional status and reduced need for diuretics and annual hospitalization. Additionally, ARNi usage has been associated with improved nutritional status, decreased reliance on diuretics, and reduced frequency of annual hospitalizations. These effects were associated with a lack of significant increase in adverse effects. These results may contribute to a better understanding of ARNi’s long-term effects on patient outcomes.
Keywords: angiotensin receptor neprilysin inhibitor, heart failure with reduced ejection fraction, sacubitril/valsartan
1. Introduction
Heart failure (HF) is a prevalent global epidemic affecting approximately 64 million individuals worldwide, with its prevalence steadily increasing.[1] According to the American Heart Association’s 2020 heart disease and Stroke Statistics Update, there is a persistent upward trend in the prevalence of individuals diagnosed with HF. Thus, by the year 2030, the prevalence of this disease is expected to increase by approximately 46%.[2] In Turkey, the HAPPY trial estimated a prevalence rate of 2.9% for HF within the Turkish population.[3]
Patients diagnosed with HF with reduced ejection fraction (HFrEF) are known to be associated with high rates of morbidity and mortality.[4] Despite the notable progress in the medical interventions and preventative strategies, there persists a considerable incidence of both mortality and morbidity, coupled with a decline in the quality of life in patients with HFrEF.[5,6] The progression of HFrEF has been greatly improved by therapies for neurohormonal antagonism through the use of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB).[7] An important step forward in this regard was achieved by the introduction of sacubitril/valsartan, an angiotensin receptor neprilysin inhibitor (ARNi) in the PARADIGM-HF trial.[8] This trial demonstrated that sacubitril/valsartan is more effective than enalapril in reducing the rates of cardiovascular (CV) death or hospitalization. As a result, the clinical guidelines started to recommend switching the use of ACEi/ARB to ARNi in HFrEF patients.[9,10] Similar findings were observed in the 1-year follow-up of HFrEF patients in the ARNi-TR study conducted in Turkey.[11] However, there is still limited knowledge regarding the long-term safety and efficacy of this treatment in routine practice. In our study we aimed to determine the long-term efficacy and safety of ARNi use in patients with HFrEF through a 5-year retrospective analysis.
2. Material and Methods
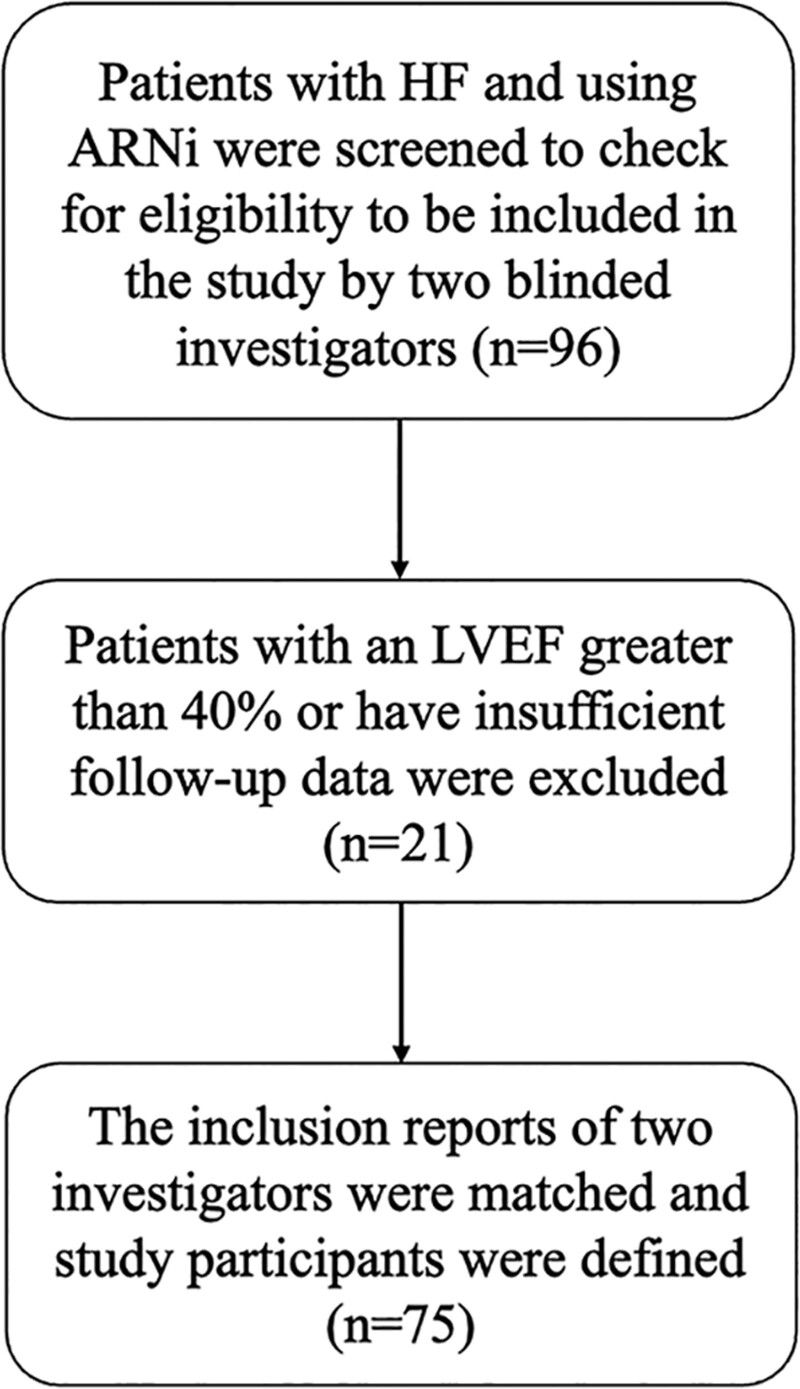
In this retrospective cohort study, a total of 96 patients diagnosed with HF and prescribed sacubitril/valsartan were screened over a 5-year period starting from January 2018. Echocardiography reports were reviewed to assess the left ventricular ejection fraction (LVEF) to exclude those with LVEF > 40%. Patients with insufficient follow-up data were also excluded and hence 75 eligible patients were included in the study (Fig. 1). To avoid selection bias, participant eligibility was evaluated separately by 2 blinded cardiologists. A thorough evaluation was conducted on various serum biochemical parameters such as creatinine, estimated glomerular filtration rate (eGFR), serum potassium, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels, as well as the New York Heart Association functional class (NYHA-FC). Additionally, the annual number of hospitalizations for HF and the mortality rates were recorded. The identification of chronic diseases, such as hypertension, diabetes mellitus, and hyperlipidemia, was based on the patients prior receipt of medical treatment for these conditions. Those with a history of coronary artery disease diagnosed through angiography were considered to have ischemic heart disease as the underlying cause of HF.
Figure 1.
Flow chart of the study.
To evaluate the adverse effects associated with ARNi use, an analysis was conducted on the medical records of the patients, and data from telephone conversations were analyzed. The eGFR was calculated using the modification of diet in renal disease equation.[12] The prognostic nutritional index (PNI), an independent predictor of long-term all-cause and CV mortality in patients with HF was also calculated using the formula PNI = 10 × serum albumin (g/dL) + 0.005 total lymphocyte count (per mm3).[13] Blood pressure values obtained during hospital visits as well as home measurements were retrospectively evaluated, and hypotension was defined as having a systolic blood pressure measurement below 100 mm Hg.
All procedures were carried out in compliance with the ethical guidelines of the Helsinki Declaration. The approval from the ethical committee at Ufuk University-Faculty of Medicine was obtained (No: 23.08.17.06/03), which waived the need to obtain consent for the retrospective collection and analysis of anonymized data in this study.
2.1. Statistical analysis
The normal distribution of variables was verified using the Kolmogorov–Smirnov test. The data that followed a normal distribution were reported in the form of mean ± standard deviation, while the data that did not follow a normal distribution were reported using the median along with the interquartile range. The categorical variables were represented in the form of percentages. The Spearman’s rho correlation coefficient was employed in cases where 1 or both of the variables exhibited non-normal distribution. The Wilcoxon signed-rank test was employed to conduct comparisons between the pre- and posttreatment periods of sacubitril/valsartan. A chi-square test was employed to examine if there were significant differences in the distributions of categorical variables across various groups. A P value of < .05 was deemed to be statistically significant. The sample size analysis was done with a confidence interval of 95% and type 1 error of 0.05. Data analysis was done using SPSS version 21.0 for windows (IBM Corp., USA).
3. Results
The mean age of the study participants was 65,73 ± 13,66 years. Baseline clinical history, comorbidities and demographic characteristics are summarized in Table 1. Most of the patients had ischemic cardiomyopathy as the etiology of HFrEF (81.3%). Most of the patients started ARNi treatment while having a NYHA-FC of III (45.3%). The rates of reaching the target dosage of 200 mg twice a day (b.i.d.) were 38.6%. The mean ejection fraction was 29,18 ± 6,94% and the mean eGFR 72,46 ± 22,72 mL/minutes per 1.73 m2. Whereas 36% of the patients had a cardiac device.
Table 1.
The baseline clinical and demographic characteristics of study population.
| Variables | Values |
|---|---|
| Age (yr) | 65,73 ± 13,66 |
| Females (%) | 21 (28%) |
| Systolic blood pressure (mm Hg) | 122.81 ± 17.13 |
| Heart rate (bpm) | 73.42 ± 11.21 |
| BMI (kg/m2) | 26,72 ± 5.35 |
| Serum creatinine (mg/dL) | 1.08 ± 0.31 |
| Potassium (mmol/L) | 4.34 ± 0.43 |
| eGFR (mL/min per 1.73 m2) | 72.46 ± 22.72 |
| NT-proBNP (pg/mL) | 3080 (1134.7–7925.2) |
| Clinical features of HF | |
| Functional class (NYHA I) (%) | 1 (1.3%) |
| Functional class (NYHA II) (%) | 29 (38.7%) |
| Functional class (NYHA III) (%) | 34 (45.3%) |
| Functional class (NYHA IV) (%) | 11 (14.7%) |
| Ischemic etiology of HF (%) | 61 (81.3%) |
| HF diagnosis time (y) | 8.2 ± 1.88 |
| Annual hospitalizations for HF (n) | 2.26 ± 0.21 |
| LVEF (%) | 29.12 ± 6.79 |
| Medical history (%) | |
| Hypertension | 47 (62.7) |
| Diabetes mellitus | 30 (40) |
| Hyperlipidemia | 56 (74.7) |
| Stroke | 4 (5.3) |
| Smoking | 13 (17.3) |
| Atrial fibrillation | 19 (25.3) |
| HF therapy (%) | |
| Beta blocker | 72 (96) |
| Previous ACEi | 42 (56) |
| Previous ARB | 21 (28) |
| MRA | 57 (76) |
| SGLT2 inh. | 35 (46,7) |
| Digital | 11 (14.7) |
| Ivabradine | 21 (28) |
| Furosemide | 55 (73.3) |
| Antiplatelet | 52 (69.3) |
| NOAC | 32 (42.6) |
| Warfarin | 6 (8) |
| Device (ICD/CRT) | 27 (36) |
ACEi = angiotensin-converting enzyme inhibitor, ARB = angiotensin receptor blockers, ARNi = angiotensin receptor neprilysin inhibitor, BMI = body mass index, BPM = beats per minute, CRT = cardiac resynchronization therapy, eGFR = estimated glomerular filtration rate, HF = heart failure, ICD = implantable cardioverter defibrillator, LVEF = left-ventricle ejection fraction, MRA = mineralocorticoid receptor antagonists, NOAC = non vitamin K oral anticoagulant, NT-proBNP = N-terminal pro-brain natriuretic peptide, NYHA = New York Heart Association, SGLT2-inh = sodium/glucose cotransporter 1 inhibitors.
Changes in patients biochemical parameters, NT-proBNP and PNI levels, LVEF values, annual hospital stays, functional capacities, diuretic doses, and ARNi doses over time are summarized in Table 2. After 5 years of ARNi use, serum NT-proBNP, and LVEF significantly improved with rare side effects that necessitated discontinuation of ARNi treatment. When gender-based comparison was conducted, no significant differences were observed between males and females regarding the changes in NT-proBNP, LVEF, and NYHA-FC following ARNi therapy initiation.
Table 2.
Changes in the clinical and laboratory parameters of heart failure over five years of ARNi administration.
| Parameters | Baseline | Fifth year | P value |
|---|---|---|---|
| ARNi dosage (twice a daily) (%) | |||
| Cessation | N/A | 12 (16%) | P < .001 |
| 50 mg | 51 (68%) | 11 (14,7%) | |
| 100 mg | 24 (32%) | 21 (28%) | |
| 200 mg | 0 (0,0%) | 31 (41,3%) | |
| Potassium (mmol/L) | 4.34 ± 0.43 | 4.38 ± 0.45 | P = 0.6 |
| Creatinine (mg/dL) | 1.08 ± 0.31 | 1.14 ± 0.35 | P = .057 |
| eGFR (mL/min per 1.73 m2) | 72.46 ± 22.72 | 67.91 ± 21.83 | P = .019 |
| PNI | 36.42 ± 15.78 | 38.28 ± 15.85 | P = .077 |
| NT-proBNP (pg/mL) | 3404 (1750–11,956) | 858 (270–2072) | P < .001 |
| Ejection fraction (%) | 29.12 ± 6.79 | 38.04 ± 7.21 | P < .001 |
| Number of annual hospitalizations | 2.26 ± 0.21 | 0.58 ± 0.11 | P < .001 |
| Functional class (NYHA) (%) | |||
| I | 1 (1.3%) | 21 (28%) | P < .001 |
| II | 29 (38.7%) | 42 (56%) | |
| III | 34 (45.3%) | 7 (9.3%) | |
| IV | 11 (14.7%) | 5 (6.7%) | |
| Dose of furosemide use (mg) | 40 (0–40) | 20 (0–40) | P < .001 |
ARNi = angiotensin receptor neprilysin inhibitor, eGFR = estimated glomerular filtration rate, NT-proBNP = N-terminal pro-brain natriuretic peptide, NYHA = New York Heart Association, PNI = prognostic nutritional index.
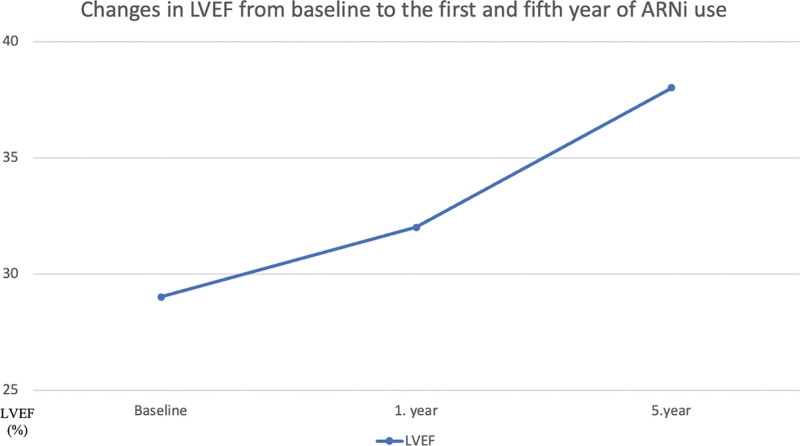
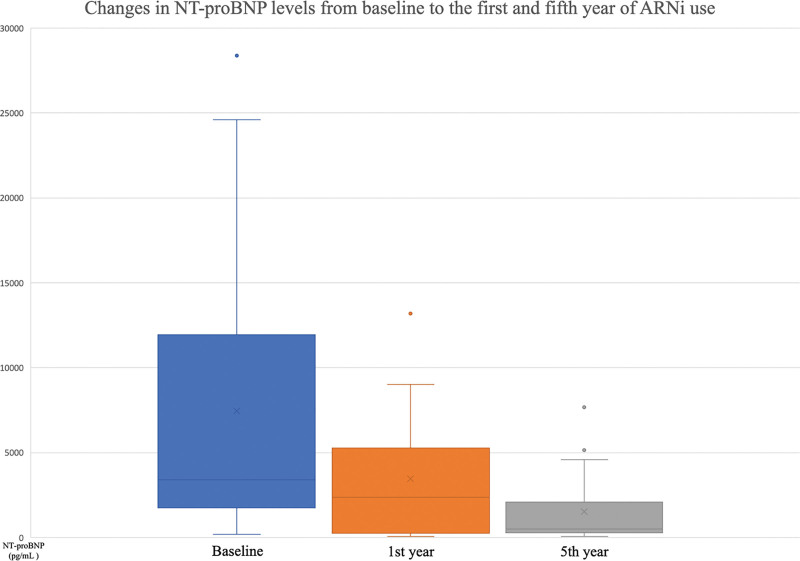
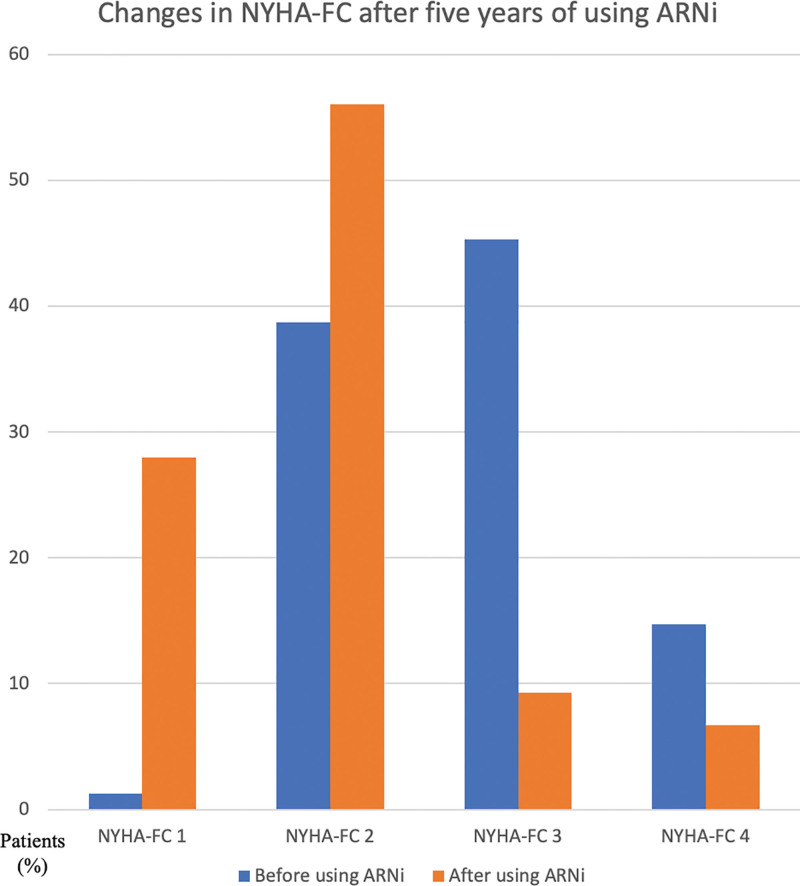
Throughout the duration of the study, beginning with the baseline measurements, the first year, and continuing until the end of the fifth year, LVEF demonstrated a steady increase, whereas NT-proBNP demonstrated a gradual decline (Figs. 2 and 3). There was also a significant shift towards lower NYHA-FC classes after 5 years of using ARNi (Fig. 4). Despite being statistically insignificant, an increase in the PNI levels was observed. Furthermore, significant reductions in the number of annual hospitalizations and daily furosemide usage doses for HF were noted. The 5-year all-cause mortality rate was found to be 26.6% in the study population, with CV deaths accounting for 17.3%. Hypotension was the most common side effect (total, 16%; symptomatic, 4%). There was a mild increase in serum potassium and creatinine levels and a mild decrease in eGFR levels (Table 2). Angioedema-like clinical status was not reported in any patient. Symptomatic hypotension (4%) and economic difficulties (11.9%) were the main reasons for the discontinuation of the drug.
Figure 2.
Changes in LVEF (%) from baseline to the first and fifth year of ARNi use. ARNi = angiotensin receptor neprilysin inhibitor, LVEF = left ventricular ejection fraction.
Figure 3.
Changes in NT-proBNP levels from baseline to the first and fifth year of ARNi use. ARNi = angiotensin receptor neprilysin inhibitor, NT-proBNP = N-terminal prohormone of brain natriuretic peptide.
Figure 4.
Changes in NYHA-FC after five years of using ARNi. ARNi = angiotensin receptor neprilysin inhibitor, NYHA-FC = New York Heart Association functional classes.
4. Discussion
This study aimed to assess the prolonged effects of ARNi usage in patients with HFrEF. The study findings align with previous shorter-term studies in the literature, indicating that the positive effects of ARNi use on LVEF, NT-proBNP, NYHA-FC and rates of annual hospitalization continued during the 5 years of follow-up without a significant increase in drug related side effects. Furthermore, the patients nutritional status, assessed by the PNI levels, demonstrated a notable improvement.
Neprilysin, also known as neutral endopeptidase, is responsible for the degradation of various vasoactive peptides, including atrial natriuretic peptide and brain natriuretic peptide.[14] Sacubitril, a neprilysin inhibitor, effectively prevents the enzymatic degradation of natriuretic peptides, thereby counteracting the excessive activation of neurohormones leading to various effects, including increased diuresis, natriuresis, and vasodilation. Thereby, the simultaneous inhibition of the renin-angiotensin system and neprilysin causes a more effective neurohormonal regulation compared to the sole inhibition of renin-angiotensin system.[15] These effects were clearly presented in the PARADIGM-HF study and subsequent real-world data has further supported these findings with the integration of ARNi into clinical practice.[8,16] Based on these findings, the recent clinical guidelines recommended the use of sacubitril-valsartan as a first-line therapy without the requirement for prior treatment with an ACEi or ARB.[17–19] Despite these facts, the ideal utilization of these drugs on long terms can present certain challenges because of the limited data of long-term ARNi use as well as the relatively high costs of this treatment in low-income communities.
In the PARADIGM-HF study, the use of ARNi was predominant in patients with FC-II (71.6%) and least commonly in those with FC-IV (0.8%). While, in our study the highest rates of ARNi use were seen in FC-III patients (45.3%), and the rate in those with FC-IV was relatively high (14.7%). The rates of ARNi use in patients with FC-III and FC-IV were even higher in in the ARNi-TR study (56.2% and 18.9%, respectively). This variation in ARNi use in the ARNi-TR study was attributed to economic factors, as the Turkish Social Security Institution does not reimburse the drug for clinical use and the patients bear the costs of the drug, resulting in a strategy of reserving ARNi for individuals with more advanced symptoms. Although our study was conducted in Turkish cities with higher socioeconomic status and a more liberal use of ARNi was expected, the prevalence of starting ARNi in patients with FC-II remained low (38.7%).
The proportion of patients in our study who were initially prescribed a low dose of ARNi (50 mg b.i.d.) was lower than that in the ARNi-TR population (68% vs 73.9%), but this rate was still relatively high as compared to the starting doses of the PARADIGM-HF study. Furthermore, despite the current guidelines advocating for the administration of ARNi at a maximum tolerated dosage of 200 mg b.i.d., the ARNi-TR study revealed that only in a minority of patients, specifically 25.6%, have reached this dosage. Over the course of our study’s 5-year follow-up period, the mentioned percentage exhibited a significant increase among our patients and ultimately reached a rate of 41,3%. Compared to that, the PROVE-HF study reported a higher rate of approximately 65% for achieving the maximum dosage.[20] The reason behind the low starting doses in our trial can be attributed to physicians tendency to initiate the treatment at lower doses because of the higher prevalence of side effects, such as hypotension, in patients with advanced heart failure, who constitute the majority of the population in our study. Economic factors may have also influenced the decision to initiate treatment with low doses and restricted the number of patients escalating to high doses, as higher doses are associated with increased costs.
In accordance with the literature and upon completion of the 5-year follow-up period in our study, the use of ARNi was associated with a significant shift towards lower NYHA-FC classes. Additionally, there was also a significant decrease in NT-proBNP levels. The PROVE-HF trial provided evidence of the beneficial effects of ARNi on cardiac remodeling.[20] The study demonstrated significant improvements in LVEF values at both the 6th and 12th months of follow-up. In our study, we also observed a notable improvement in LVEF measures after 1-year of ARNi use which was further improved during the subsequent follow-up period. Specifically, the mean LVEF value rose significantly from 29.2 ± 6.9% to 38.04 ± 7.21% at the end of the 5 years of follow-up. Furthermore, the SAVE-implantable cardioverter defibrillator (ICD) trial, showed that the improvement in LVEF after 6 months of ARNi treatment potentially obviated the need for ICD implantation in nearly 1 out of 4 patients.[21] We also observed a lower incidence of cardiac device implantation compared to the ARNi-TR study (27.1% vs 39.2%), suggesting that more prolonged use of ARNi yields sustained effects on LVEF, thereby reducing the necessity for cardiac device implantation. Hence, the long-term applicability of the findings from the SAVE-ICD trial was observed.
In parallel with the previous studies, there was a notable reduction in the annual hospitalization rates for HF following the extended administration of ARNi. Furthermore, a notable reduction in the administration of diuretics was observed, with some instances even resulting in complete cessation. The observed decrease in furosemide dosage among our patients can be attributed to the natriuretic properties of sacubitril or the anticipated enhancement in hemodynamic conditions. On the other hand, PNI levels which reflects the patients’ nutritional status and considered to be a predictor of mortality in HF patients was higher at the end of the follow-up period.[13]
Regarding mortality rates, our study revealed a 5-year CV mortality rate of 17.3%, which was found to be higher than that reported in the PARADIGM-HF study (13.3%). The higher rate of CV mortality observed in our study can be explained by many factors, such as the advanced stage of heart failure in our study population, a prolonged duration of follow-up, and a lower prevalence of attaining the maximum doses of ARNi.
The most common adverse event observed in our study was hypotension, with an overall incidence rate of 16%, 4% of these cases were symptomatic. In the ARNi-TR trial, the reported incidence of symptomatic hypotension was 2.3%, whereas in the PARADIGM-HF trial, it was 14%. This issue holds significant importance for patients who are being considered for the initiation of ARNi therapy, but present with low BP. A more gradual titration process and close monitoring can enhance drug tolerability in patients who are considered to be more physically delicate. On the other hand, reducing or discontinuing the dosage of other BP lowering medications in such patients may enhance the tolerability of ARNi therapy. Other expected adverse effects of ARNi includes the disturbances in the renal function and electrolyte balance. A statistically significant increase in serum potassium and creatinine levels and a decrease in eGFR have been reported in the ARNi-TR study, contrarily at the end of the 5 years follow-up of our study, the increase in mean serum potassium and creatinine levels and the decrease in mean eGFR were statistically insignificant. These findings suggest that, despite the higher prevalence of high dosage utilization of ARNi in our study as compared to the ARNi-TR trial, patients may exhibit increased tolerance towards the drug over an extended period of usage. Our study did not report the occurrence of angioedema, which is considered a rare complication with a prevalence rate of 0.4% as reported in the PARADIGM-HF trial.
In our study, the main factors contributing to ARNi discontinuation were symptomatic hypotension and economic considerations. However, severe renal dysfunction was not acknowledged as a reason for drug cessation. Improved patient outcomes and reduced severity of side effects are associated with higher levels of treatment compliance. Consistent with this notion, our study findings showed that the drug exhibited greater efficacy and fewer adverse effects during the long-term follow-up, potentially leading to improved patient adherence to the medication. In the light of that, maximizing the therapeutic benefits of ARNi utilization specially during the early phases of HF should be enhanced. This recommendation is further supported by the growing expertise and familiarity with the drug administration. In addition, the inability to achieve the desired maximum dosage of 200 mg b.i.d. can be addressed by demonstrating a greater willingness and caution in gradually escalating the dosage. The retrospective design of this study was a major limitation, which resulted in restricted data availability in certain instances.
5. Conclusion
The known short-term effects of ARNi use in patients with HFrEF are further evident on the long-term. This manifested as the improvements in NYHA-FC, LVEF, and NT-proBNP levels, while also leading to a better nutritional status and a reduced need for diuretics and annual hospitalization. Furthermore, the long-term use was associated with a lack of a significant increase in the incidence side effects incidence and thus high levels of drug adherence. Nevertheless, it is essential to enhance the use of ARNi in the early stages of HF while aiming at the highest tolerated doses in order to maximize the therapeutic benefits of this medication.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Author contributions
Conceptualization: Ajar Koçak, Berkay Ekici.
Data curation: Ajar Koçak, Saadet Aydin, Hayrudin Alibaşiç, Melis Çiçek.
Formal analysis: Ajar Koçak, Saadet Aydin, Hayrudin Alibaşiç, Melis Çiçek, Berkay Ekici.
Funding acquisition: Ajar Koçak, Saadet Aydin, Hayrudin Alibaşiç, Melis Çiçek, Berkay Ekici.
Investigation: Ajar Koçak, Melis Çiçek, Berkay Ekici.
Methodology: Ajar Koçak, Berkay Ekici.
Project administration: Ajar Koçak, Hayrudin Alibaşiç, Berkay Ekici.
Resources: Ajar Koçak, Saadet Aydin, Hayrudin Alibaşiç, Melis Çiçek.
Software: Ajar Koçak, Saadet Aydin, Melis Çiçek.
Supervision: Saadet Aydin, Hayrudin Alibaşiç, Berkay Ekici.
Validation: Saadet Aydin, Hayrudin Alibaşiç, Berkay Ekici.
Visualization: Ajar Koçak, Saadet Aydin, Hayrudin Alibaşiç, Berkay Ekici.
Writing – original draft: Ajar Koçak.
Writing – review & editing: Ajar Koçak, Saadet Aydin, Berkay Ekici.
Abbreviations:
- ACEi
- angiotensin-converting enzyme inhibitors
- ARB
- angiotensin receptor blockers
- ARNi
- angiotensin receptor neprilysin inhibitor
- CV
- Cardiovascular
- eGFR
- estimated glomerular filtration rate
- HF
- heart failure
- HFrEF
- heart failure with reduced ejection fraction
- ICD
- implantable cardioverter defibrillator
- LVEF
- left ventricular ejection fraction
- NT-proBNP
- N-terminal prohormone of brain natriuretic peptide
- NYHA-FC
- New York Heart Association functional classes
- PNI
- prognostic nutritional index
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Koçak A, Aydin S, Alibaşiç H, Çiçek M, Ekici B. Long-term effects of angiotensin receptor neprilysin inhibitor therapy in heart failure patients with reduced ejection fraction: A retrospective cohort study. Medicine 2023;102:43(e35589).
Contributor Information
Saadet Aydin, Email: dr.saadetaydin@gmail.com.
Hayrudin Alibaşiç, Email: dr.halibasic@gmail.com.
Melis Çiçek, Email: drmeliscicek@gmail.com.
Berkay Ekici, Email: berkay.ekici@gmail.com.
References
- [1].Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology [published correction appears in Cardiovasc Res 2023 Jun 13;119(6):1453]. Cardiovasc Res. 2023;118:3272–87. [DOI] [PubMed] [Google Scholar]
- [2].Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–596. [DOI] [PubMed] [Google Scholar]
- [3].Değertekin M, Erol C, Ergene O, et al. Heart failure prevalence and predictors in Turkey: HAPPY study. Turk Kardiyol Dern Ars. 2012;40:298–308. [DOI] [PubMed] [Google Scholar]
- [4].Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lupón J, Díez-López C, de Antonio M, et al. Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. Eur J Heart Fail. 2017;19:1615–23. [DOI] [PubMed] [Google Scholar]
- [6].Greenberg B. Medical management of patients with heart failure and reduced ejection fraction. Korean Circ J. 2022;52:173–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Metra M, Teerlink JR. Heart failure. Lancet. 2017;390:1981–95. [DOI] [PubMed] [Google Scholar]
- [8].McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- [9].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- [10].Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–61. [DOI] [PubMed] [Google Scholar]
- [11].Ekici B, Yaman M, Küçük M, et al. Angiotensin receptor neprilysin inhibitor for patients with heart failure and reduced ejection fraction: real-world experience from Turkey (ARNi-TR). Turk Kardiyol Dern Ars. 2021;49:357–67. [DOI] [PubMed] [Google Scholar]
- [12].Imai E, Horio M, Nitta K, et al. Modification of the modification of diet in renal disease (MDRD) study equation for Japan. Am J Kidney Dis. 2007;50:927–37. [DOI] [PubMed] [Google Scholar]
- [13].Turen S, Memic Sancar K. Predictive value of the prognostic nutritional index for long-term mortality in patients with advanced heart failure. Acta Cardiol Sin. 2023;39:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Volpe M, Carnovali M, Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci (Lond). 2016;130:57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail. 2014;2:663–70. [DOI] [PubMed] [Google Scholar]
- [16].Aimo A, Pateras K, Stamatelopoulos K, et al. Relative efficacy of Sacubitril-Valsartan, Vericiguat, and SGLT2 inhibitors in heart failure with reduced ejection fraction: a systematic review and network meta-analysis. Cardiovasc Drugs Ther. 2021;35:1067–76. [DOI] [PubMed] [Google Scholar]
- [17].Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79:e263–421. [DOI] [PubMed] [Google Scholar]
- [18].McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- [19].McDonagh TA, Metra M, Adamo M, et al. 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627–39. [DOI] [PubMed] [Google Scholar]
- [20].Januzzi JL, Jr, Prescott MF, Butler J, et al. Association of change in N-terminal pro-b-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction (PROVE-HF). JAMA. 2019;322:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Guerra F, Ammendola E, Ziacchi M, et al. Effect of SAcubitril/Valsartan on left v entricular ejection fraction and on the potential indication for implantable cardioverter defibrillator in primary prevention: the SAVE-ICD study. Eur J Clin Pharmacol. 2021;77:1835–42. [DOI] [PubMed] [Google Scholar]