Abstract
During endotoxin-induced Acute Lung Injury (ALI), immune cell recruitment resulting from chemotaxis is mediated by CXC and CC chemokines and their receptors. In the current study, we investigated the role of chemokines and their receptors in the regulation of myeloid cell populations in the circulation and the lungs of C57BL/6J mice exhibiting lipopolysacchride (LPS)-mediated ALI using Single Cell RNA sequencing (scRNA-seq). During ALI, there was an increase in the myeloid cells, M1 macrophages, monocytes, neutrophils, and other granulocytes, while there was a decrease in the residential alveolar macrophages, and M2 macrophages. Interestingly, LPS triggered the upregulation of CCL3, CCL4, CXCL2/3, and CXCL10 genes associated with cellular migration of various subsets of macrophages, neutrophils, and granulocytes. Furthermore, there was an increase in the frequency of myeloid cells expressing CCR1, CCR3, CCR5, and CXCR2 receptors during ALI. miRNAseq studies of Vehicle vs LPS groups identified several dysregulated miRNAs targeting the upregulated chemokine genes. The current study suggests that chemokine ligand-receptors interactions are responsible for myeloid cell heterogenicity and cellular recruitment to the lungs during ALI. The single-cell transcriptomics allowed for an in-depth assessment and characterization of myeloid cells involved in immune cell trafficking during ALI.
Introduction
The attraction of leukocytes to the infectious site is moderated by a unique family of secretory proteins referred to as chemokines (1, 2). Chemokines can be divided primarily into two groups consisting of CXC and CC family of chemokines. The differences in amino acid sequences of the CXC and CC family of chemokines separate the two families. CXC chemoattractants are a group of chemokines in which the first two cysteines are separated by a single amino acid, while in CC chemokines, the first two cysteines, are adjacent. However, both families and their associated receptors stimulate the chemotaxis of neutrophils, monocytes, macrophages, T cells, dendritic cells, eosinophils, B cells, and natural killer (NK) cells (3) . Interestingly, there is redundancy amongst several chemokines and chemokine receptors (4). The chemokine receptors are a group of G-protein-couple receptors with varying levels of binding specificity to different chemokines. CXC chemokines bind to CXCR chemokine receptors, whereas CC chemokines bind to CCR signaling receptors (3).
During host inflammatory response to a pathogen, chemotaxis orchestrates the migration of immune cells by moving them along a chemical gradient. Trafficking of leukocytes to the site of infection is regulated by chemokine-driven ligand-receptor interactions (3, 5). The changes occurring during the host immune cell activation and defense during homeostatic and pathogenic conditions are partially dependent on the chemokine ligand-receptor axis. In addition to the activated immune cells, injured host-derived cells release cytokines and chemokines into circulation that bind the specific receptors on effector immune cells, which direct the cells’ movement to this site of inflammation (3, 6, 7).
LPS-induced Acute Lung Injury (ALI) in the mouse is a model regulated by chemokine ligand-receptors recruitment of leukocytes (8). In ALI, the migration of CCR2+ monocytes and CXCR2+ neutrophils to the sites of lung injury is orchestrated by the chemoattractant CCL2 and CXCL2/3, respectively (9–12) . However, the emigration of additional myeloid cell subsets to the lungs, and the specific chemokines and chemokine receptors involved during ALI is not clear.
In this study, we examined the recruitment of myeloid cell subsets during LPS-induced ALI by studying myeloid cell-associated CCL and CXCL ligands and their associated receptors. We used both flow cytometry-based immune cell-profiling and single-cell RNA sequencing to better understand the role of chemokines and their receptors in the recruitment of myeloid cells in the lungs. The current study identifies the chemokine ligand-receptors interactions responsible for myeloid cell heterogenicity and cellular recruitment to the sites of lung injury.
Materials and Methods
Mice
C57BL/6 female mice, 8–10 weeks of age, were purchased from Jackson Laboratories [Bar Harbor, ME, USA]. Mice were kept under 12-hour light/12-hour dark cycles in temperature-controlled rooms and specific pathogen-free conditions at the AAALAC-accredited animal facility located at the University of South Carolina School of Medicine [Columbia, SC]. Mice were given water and a standard chow diet as often as necessary.
Induction of ALI and isolation of cells from the blood and lungs
To induce ALI, mice were given 20 ul of LPS (2mg/kg) or phosphate-buffered saline (PBS) intranasally. The LPS (Escherichia coli, serotype 055:B5) was purchased from Sigma-Aldrich, St. Louis, MO. The control mice received the vehicle only. After 48 hours, mice were euthanized, and whole blood was collected from the portal vein and resuspended in 10 ul EDTA and 2 mL of red blood lysis for 10 minutes and neutralized with 10 mL flow cytometry staining buffer (FACS buffer-1X PBS, 2% Fetal Bovine Serum (FBS), and 2mM EDTA). Subsequently, cells were centrifuged at 300 g for 10 min at 4°C and resuspended in FACS. Animals were then perfused with 10 mL of heparinized PBS. Lungs were excised, placed in 5 ml of FACS, homogenized into a single cell solution, and filtered with a 40uM strainer. Cells were centrifuged at 300 g for 10 min at 4°C. The supernatant was discarded, and the cell pellet was resuspended in 1 mL of red blood cell lysis buffer and placed on ice for 5 minutes. The single-cell solution was then neutralized with 5 ml of FACS buffer and centrifuged at 300 g for 5 min at 4°C. After discarding the supernatant, cells were washed and centrifuged twice more. The cell pellet was resuspended in 5 ml FACS buffer and filtered. We used the Bio-Rad TC20 Automated Cell Counter [Hercules, CA] to count cells in the lung and the blood.
Flow cytometry and sorting
Flow cytometry analysis was performed on lung cells as described by Mohammed et al. 2020 (13). In brief, cell solution was diluted to 1× 106 cells/ml for flow cytometry analysis and incubated with TruStain FcX anti-mouse CD16/32 [Biolegend, San Diego, CA] for 10 minutes at room temperature. Next, the cells were incubated with fluorescently labeled monoclonal antibodies (mAbs) anti-CD45 (APC/Cy7), anti-CD11b (AF700; BV786), anti-CD11c (BV786; BV605), anti-F4/80 (BV421; AF488), anti-Gr-1 or anti-Ly6G (BV-510), anti-Ly6C (AF488), anti-CCR5 (PerCP), anti-CCR2 or anti CXCR5 (FITC), anti-CCR3 (AF647), anti-CCR1 or anti-CXCR2 (P.E.), and/or anti-CD206 (BV650), purchased from Biolegend, for 30 minutes on ice. Subsequently, cells were washed with 1 mL FACS buffer and centrifuged at 300 g for 10 min at 4°C, three times. After the final wash, cells were resuspended in 600ul of FACS buffer. Samples were analyzed using BD FACS Celesta and FlowJo v10 software [Ashland, OR]. All samples were gated on FSC-H, SSC-H, and CD45. Afterward, samples were gated on CD11b and respective chemokine receptors. Furthermore, flow plot heatmaps were analyzed to identify whether the populations were also positive for CD11c, Gr-1, Ly6G, or Ly6C.
Single Cell RNA Seq
As described by Becker et al. 2020 (14), the 10x Chromium instrument [10x Genomics, Pleasanton, California] was used to execute single-cell RNA sequencing. Three-thousands cells per sample were loaded on the Chromium Controller [10x Genomics] to generate single-cell Gel Bead-In-Emulsions. Next, RNA-seq libraries were generated using the Chromium v2 single-cell 3′ RNA-seq reagent kit [10x Genomics], and sequenced with NextSeq 550 instrument [Illumina, San Diego, CA]. Next, raw files were processed using Cell Ranger version 3.1.0 [10x Genomics], and downstream analysis of Cell Ranger output was completed using Loupe Browser 5.1.0.
microRNA Sequencing
For RNA extraction and quantification, total RNA was extracted from samples with 1×106 cells/mL using the MiRNeasy Mini Kit [QIAGEN, Hilden, Germany] following manufacturer’s instructions. To confirm the concentration of RNA, samples were quantified using the Qubit™ High Sensitivity RNA Assay Kit [Thermo Fisher, Waltham, MA]. Furthermore, for library preparation and sequencing, libraries were prepared with 100 ng of miRNeasy RNA using the QIAseq miRNA Library Kit [QIAGEN] following the protocol as provided by the manufacturer. The concentrations for each library were measured using the Qubit™ dsDNA HS Assay Kit [Thermo Fisher]. Sizes for each library was quantified utilizing a 1.5% Agarose gel. Libraries were then sequenced on a NextSeq 550 Sequencer [Illumina], with version 2 chemistry. Primary and secondary analysis was performed using the GeneGlobe Web-Based Analysis tool [QIAGEN]. FASTQ files were uploaded to the GeneGlobe tool and data was aligned and hit counts created using the RNA-seq Analysis Portal. Samples were then queued for secondary analysis in which miRNAs from all samples were normalized and fold change resolved. Differentially detected miRNAs between samples were illustrated in multiple plots and charts. miRNAs with FDR p-values of <0.05 and fold change of > 1.5 or < −1.5 were selected for further investigation.
RT-qPCR to determine the expression of CCL3, CXCL2, and CXCL3 in lung MNCs.
To determine the expression of CCL3, CXCL2, and CXCL3 in lung infiltrating mononuclear cells (MNCs), lung MNCs were harvested using Histopaq-1119 and histopaq-1077 purchased from Sigma-Aldrich (St. Louis, MO) and following the protocol of the company. In brief, 3 ml of Histopaq-1119 was added in a 15 ml conical centrifuge tube and then carefully 3 ml of histopaq-1077 was added onto histopaq-1119. Next, 6 ml of cells harvested from the lungs were added onto the upper gradient of the tube. The column with lung cells was centrifuged at 700 g for 20 minutes at room temperature. The upper layer above the histopaq-1077, was carefully removed. Close to the histopaq-1077, lung MNCs were collected and washed twice with complete medium. Lung MNC cells were finally suspended in complete medium and their numbers were counted.
Lung MNCs (2×106/ml) were next transferred to a 12-well plate and were cultured in the presence of vehicle, IL-6 (10 ng/ml), LPS (10 ng/ml), and IL-6 (10 ng/ml) + LPS (10 ng/ml) for 18 hours at 37°C, 5% CO2. The mouse recombinant IL-6 was purchased from Bio Legend (Cat# 575702). The cells were collected and washed twice with cold PBS. The pellet was then suspended in lysis buffer from RNeasy mini kit from Qiagen and total RNA was isolated using RNeasy mini kit from Qiagen as indicated in the protocol of the company. RT-qPCR was performed using cDNA generated from total RNA isolated from the lung MNCs. Mouse CCL3-, CXCL2-, and CXCL3-specific primers were used to determine the expression of CCL3, CXCL2, and CXCL3. The details of the primers are as given below. Mouse 18S primers were used as an internal control. The RT-qPCR was performed using kit from Applied Biosystems on cDNAs generated from lung MNCS. The following conditions were used to perform RT-qPCR: 40 cycles for 5 min at 95°C (initial activation step), 15 s at 94°C, 30 s at 60°C, and 45 s at 72°C. The value of genes was normalized against the housekeeping gene (18S) and fold change of gene expression was calculated against 18S and the treated groups.
Details of Primers:
Mouse 18S Forward Primer: 5’-GCCCGAGCCGCCTGGATAC-3’
Mouse 18S Reverse Primer: 5’-CCGGCGGGTCATGGGAATAAC-3’
Mouse CCL3 Forward Primer:5’-TGACACTCTGCAACCAAGTC-3’
Mouse CCL3 Reverse Primer: 5’CGATGAATTGGCGTGGAATC-3’
Mouse CXCL2 Forward Primer:5’-TCAATGCCTGAAGACCCTG-3’
Mouse CXCL2 Reverse Primer: 5’-CCTTGAGAGTGGCTATGACTTC-3’
Mouse CXCL3 Forward Primer: 5’-TTTGAGACCATCCAGAGCTTG-3’
Mouse CXCL3 Reverse Primer: 5’-CCTTGAGAGTGGCTATGACTTC-3’
Preparation of bronchoalveolar lavage fluid (BALF) and detection of cytokines using ELISA:
Cytokines in the BALF were obtained as described previously (15). Briefly, 48 h after vehicle or LPS exposure, mice were euthanized. The trachea was bound with a suture and the lung was excised as an intact organ along with the bound trachea. Sterile ice-cold PBS was injected through the trachea and the instilled fluid is gently retracted to maximize BAL fluid retrieval. Cytokine detection was carried out using ELISA. To detect cytokine levels, BALF was centrifuged at 2500 g for 12 minutes, afterwards, supernatant was collected and 100 μl of BALF supernatant was used to detect cytokine levels using ELISA kits purchased from Biolegend, San Diego, California.
Statistical analyses
Student’s t-test was used for statistical analyses. The number of mice used has been presented as individual dots on the bar graphs. All data shown in figure legends are presented as Mean ± standard error of the mean [SEM]. The levels of statistical significance were determined using the following: *p < 0.05, **p < 0.01, ***p < 0.001, ****p<0.0001. For the analysis of data generated by RT-qPCR, ANOVA was performed using GraphPad version 6.0 (GraphPad Software, Inc., San Diego, CA) to determine the significant differences in the expression of genes, and differences between the groups were considered significant when p<0.05.
Results
LPS-induced ALI enriches myeloid cell populations and genes associated with chemotaxis
In a recent study, we noted that LPS exposure in mice leads to an influx of infiltrating immune cells in the lungs, alveolar and bronchiolar epithelial hyperplasia, appearance of dark scar tissue associated with necrosis (anoxia), and collapsed alveolar sacs (16). Using whole-body plethysmography, we found that LPS caused an increase in the percentage of rejected breaths during lung functionally testing duration (RINX), decreased the amount of air moving in and out of the lungs during each respiratory cycle (TV), and increased the ratio of peak expiratory flow (Rpef) (16). These data suggested that LPS administration caused significant inflammation and injury in the lungs.
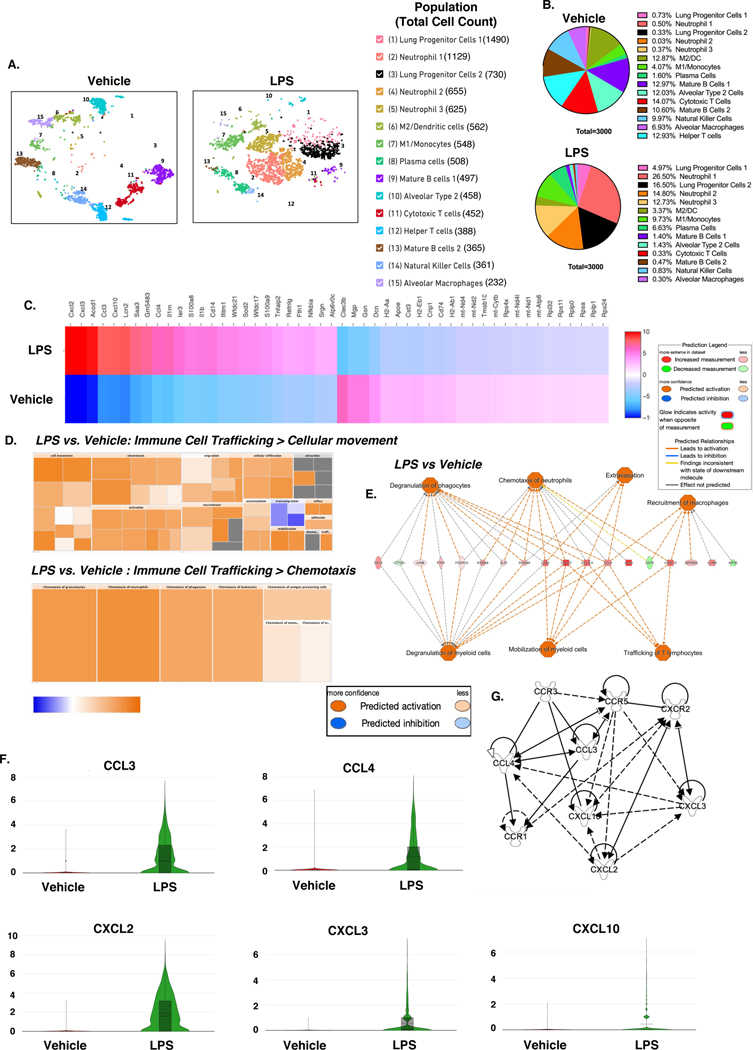
Transcriptional profiles of immune cells often highlight the activity of the cells. Therefore, to evaluate the changes in transcriptional profiles of myeloid cells in relation to immune cell trafficking during inflammation, we compared the transcripts of cells isolated from the lungs of LPS-challenged mice to the vehicle controls using scRNA-seq. The lung immune cell profiles of mice were significantly altered when mice were challenged with LPS. We observed an increase in M1 macrophages, monocytes, and granulocytic cellular clusters, such as the neutrophil subsets, plasma cells, and lung progenitor cells in LPS group vs Veh controls (Figure 1A&B). Interestingly, there was a significant decrease in certain cell populations following LPS exposure such as M2/Dendritic cells (DC), mature B cells, Alveolar type-2 cells, cytotoxic and helper T cells, NK cells, and alveolar macrophages (Figure 1B).
Figure 1. scRNA-seq analysis reveals dysregulation of chemokine genes in the lungs of mice with ALI.
Mice were exposed intranasally with LPS or the Vehicle and 48 hours later, the lungs were harvested for analysis as described in Methods. The cells from the lungs were pooled from groups of 5 mice for each of the Vehicle or LPS groups and the experiment was carried out once. A. scRNA-seq t-SNEs of lung cells. B. Pie chart of cell populations by percentages. C. Heat map of Top 50 variable genes across treatment groups. D. IPA generated heatmaps of dysregulated canonical pathways predicted to be associated with the dysregulated genes in myeloid cell clusters of Veh vs LPS mice. E. IPA graphical summary of dysregulated genes and their relationship with immune cell trafficking. F. Violin plots of dysregulated chemokine genes across treatment groups. G. IPA pathway builder generated relationships of dysregulated chemokine ligands-receptors.
Comparing the global transcriptional profile of LPS-challenge mice vs control mice using Loupe Browser, we determined that a variety of transcripts were dysregulated amongst the groups. Several of these dysregulated transcripts were genes that coded for chemokines. For example, CCL3, CCL4, CXCL2/3, and CXCL10, genes associated with cellular migration of macrophages, neutrophils, phagocytes, and granulocytes during inflammation, were upregulated in LPS group vs Veh controls (Figure 1C). When LPS vs Veh control groups were compared using Ingenuity pathway analysis (IPA), it was predicted that several of the top 50 dysregulated genes were highly associated with immune cell trafficking, cellular movement, and chemotaxis (Figure 1C&D). In addition, IPA graphical summary tool summarized the relationship between upregulated and downregulated genes across the groups, and predicted their association to be involved in immune cell trafficking. Upregulation of CCL3 and CCL4 was predicted to activate degranulation of phagocytes and myeloid cells, mobilization of myeloid cells, extravasation, and trafficking of T lymphocytes. However, CCL4, along with CXCL10, was also predicted to activate the recruitment of macrophages. CXCL10, CXCL3, and CCL3 were predicted to activate the chemotaxis of neutrophils (Figure 1E). Further investigation into CCL3, CCL4, CXCL2/3, and CXCL10 genes showed different expression levels across myeloid cell clusters. CCL3, CCL4, CXCL2, CXCL3, and CXCL10 genes were upregulated in the LPS group compared to Veh controls (Figure 1F). In addition, using the IPA pathway building tool, we were able to establish the connections between CCL3, CCL4, CXCL2/3, and CXCL10 and several CCR and CXCR receptors (Figure 1G). Together, these data demonstrated the heterogenicity of myeloid cell subsets recruited to the lungs and the key role played by the chemokines during ALI.
Chemokine gene expression disproportionately expressed across myeloid cell population during lung injury.
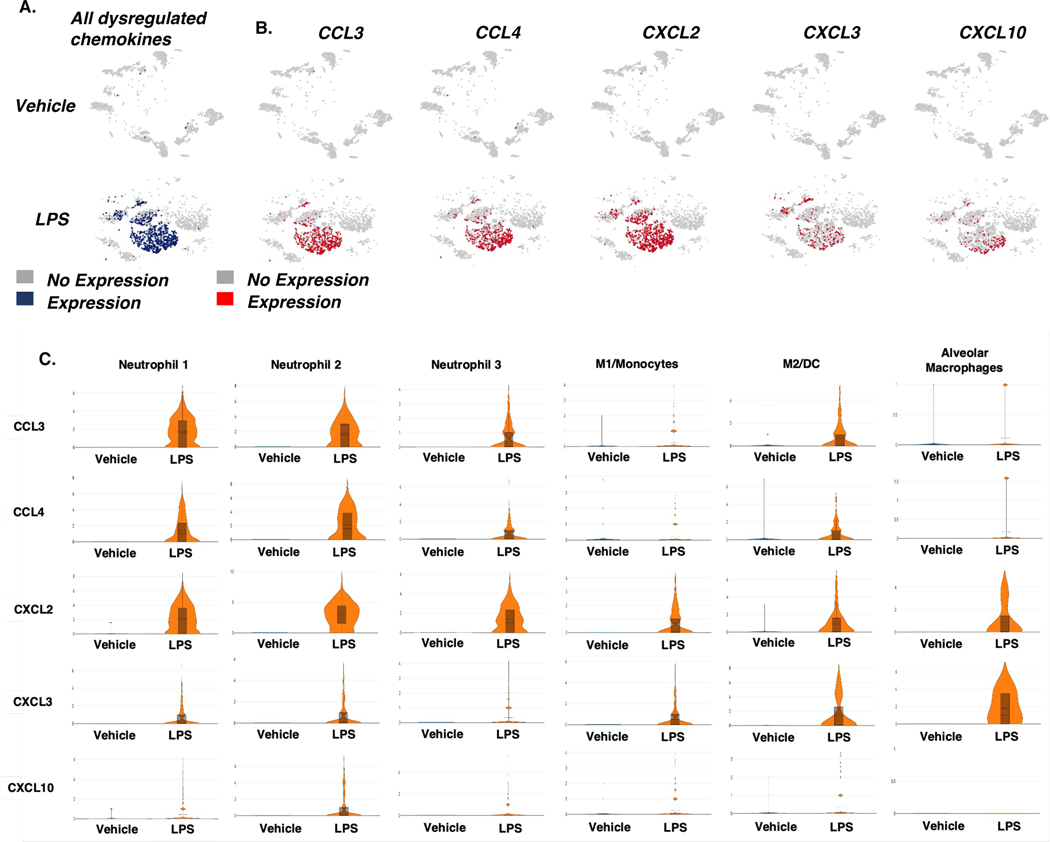
Next, we investigated the relationship between dysregulated gene expression of chemokine ligands and myeloid cell clusters. Sample ID scRNA-seq t-SNEs were generated to study gene expression of ligands CCL3/4 and CXCL2/3/10. In LPS-exposed mice, gene expression for CCL3/4 and CXCL2/3/10 was upregulated in the cells isolated from the lungs (Figure 2 A&B). CCL3/4 transcripts were primarily expressed in Neutrophil 1–3 and M2/DC clusters (Figure 2 C). CXCL2 expression was increased at different levels in Neutrophil clusters 1–3, M1/monocyte, M2/DC, and Alveolar Macrophage (AM) clusters following LPS exposure. Neutrophils 1&2, M2/DC, and M1/monocytes clusters moderately expressed CXCL3 during ALI, but the transcript was highly upregulated in AM cluster. CXCL10 was expressed at low levels by Neutrophil 1–2, M1/Mono, and M2/DC clusters during ALI (Figure 2C). Taken together, these data demonstrated that during ALI, the expression of chemokine genes involved in the trafficking of myeloid cells is significantly increased across several myeloid cell subsets.
Figure 2. Expression of dysregulated chemokine genes in cells from across treatment groups.
Mice were exposed intranasally with LPS or the Vehicle and 48 hours later, the lungs were harvested for analysis as described in Methods. The cells from the lungs were pooled from groups of 5 mice for each of the Vehicle or LPS groups and the experiment was carried out once. A. t-SNE plots of gene expression in all chemokine ligands tested in immune cells isolated from the lungs. B. t-SNE plots of gene expression in specific chemokine ligands tested in immune cells isolated from the lungs. C. Violin plots of chemokine ligand gene expression across myeloid cell populations.
Chemokine receptor expression in immune cells during ALI.
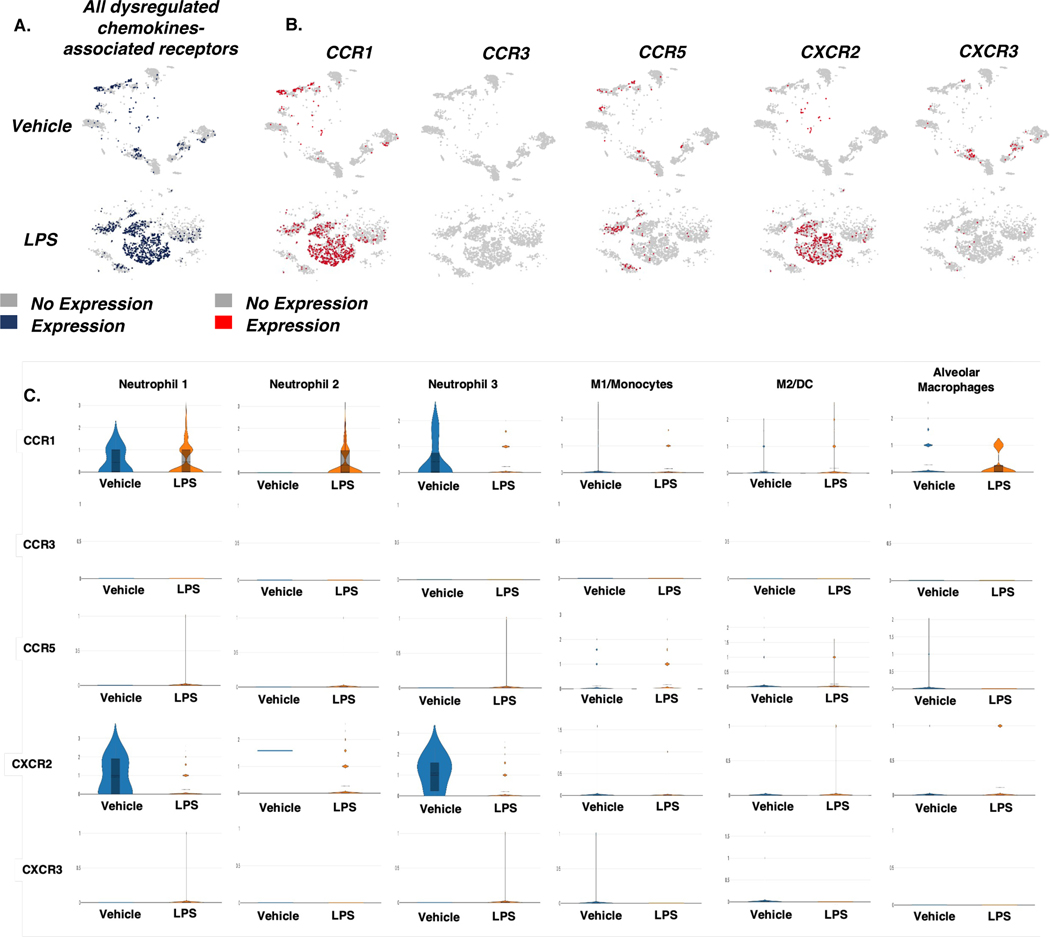
Next, we investigated the relationship between dysregulated gene expression of chemokine ligands and the associated chemokine receptors in myeloid cell clusters. To study gene expression of ligands CCL3/4 and CXCL2/3/10 and associated receptors CCR1/3/5 and CXCR2/3, sample ID scRNA-seq t-SNEs were generated (Figure 2A&B; Figure 3A&B ). The ligand CCL3 binds to the CCR1 and CCR5 receptors, while CCL4 binds to the chemokine receptors CCR1, CCR3, and CCR5. In addition, CXCL2/3 binds to the chemokine receptor CXCR2, and CXCL10 binds to the CXCR3 receptor (Figure 1G). In LPS-exposed mice, gene expression of CCR1/5 and CXCR2/3 was upregulated in the cells isolated from the lungs (Figure 3B).
Figure 3. Expression of dysregulated chemokine receptor genes in cells from across treatment groups.
Mice were exposed intranasally with LPS or the Vehicle and 48 hours later, the lungs were harvested for analysis as described in Methods. The cells from the lungs were pooled from groups of 5 mice for each of the Vehicle or LPS groups and the experiment was carried out once. A. t-SNE plots of gene expression of all chemokine receptors tested in immune cells isolated from the lungs. B. t-SNE plots of gene expression in specific chemokine receptors tested in immune cells isolated from the lung. C. Violin plots of chemokine receptor gene expression across myeloid cell populations.
Interestingly, chemokine receptor CCR1 was expressed mainly by Neutrophil 1–3, and in alveolar macrophages and to a lesser extent on M1/monocytes and M2/DC clusters. CCR3 and CCR5 were weakly expressed in various cell types tested while CXCR2 was primarily expressed in Neutrophils 1 and 3 (Figure 3 C). However, when LPS group was compared to Veh controls, CCR1 expression was upregulated in Neutrophil 2 and AM cells but not in Neutrophil 1 and 3 sub population in LPS group (Figure 3C). Also, CXCR2 was downregulated in Neutrophil 1 and 3 following LPS exposure (Figure 3C). However, it should be noted that while the expression of the chemokine receptors following LPS treatment showed slight increase or no change to downregulation in individual cell subsets tested, there was significant increase in the expression of CCR1, CCR5, and CXCR2 in the overall population of cells (Figure 3 A, B) and a dramatic increase in the proportion of Neutrophils 1, 2, and 3 as well as M1 macrophages following LPS treatment (Figure 1B), which together suggested that ALI does induce expression of chemokine receptors.
Lung injury induced enrichment of heterogenicity amongst innate immune cells in the lungs validated with flow cytometry-based immune-profiling.
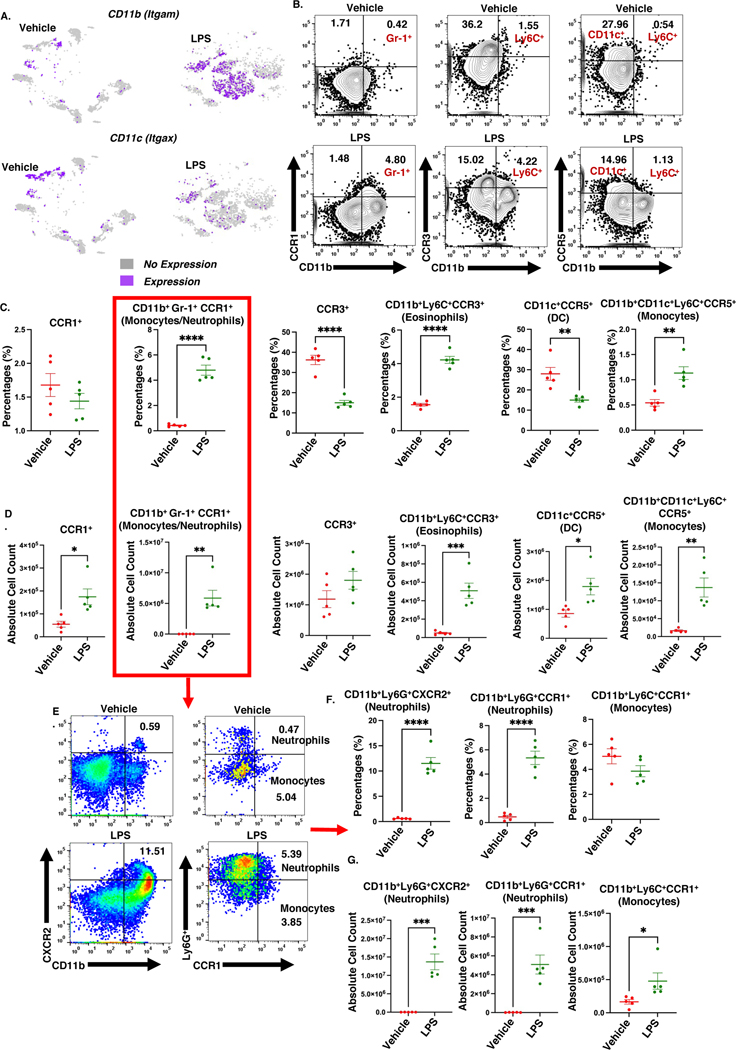
Next, we used multicolor flow cytometry to examine the immune cells phenotypes in the lungs during ALI, and correlate with signature gene profiles. Itgam and Itgax are the gene signatures of myeloid cells, that code for the cellular receptors CD11b and CD11c, respectively. CD11b is expressed by macrophages, monocytes, and neutrophils, while alveolar macrophages and dendritic cells mainly express CD11c. In mice exposed to LPS, there was an increase in myeloid cell clusters expressing Itgam and a decrease in the expression of Itgax (Figure 4A). Immunophenotyping cell analyses revealed that LPS-treated mice had a higher percentage and absolute numbers of CD11b+Gr-1+CCR1+ (neutrophils/monocytes), CD11b+Ly6C+CCR3+ eosinophils, and CD11b+CD11c+Ly6C+CCR5+ monocytes (Figure 4 C, D). On the other hand, LPS-treated mice had lower percentage but higher numbers of CD11c+CCR5+ dendritic cells (Figure 4C, D). Further investigation into the CD11b+Gr-1+CCR1+ population (Figure 4E) showed LPS-treated group had increased percentage and numbers of both CD11b+Ly6G+CCR1+ and CD11b+Ly6G+CXCR2+ neutrophils but with respect to CD11b+Ly6C+CCR1+ monocytes, LPS treatment increased their numbers but not the percentage (Figure 4 F, G). Thus, our data supported the overall findings that ALI triggers myeloid cell population heterogeneity and positive shifts in chemokine receptors cell surface expression on myeloid cells.
Figure 4. Acute lung injury triggers the heterogenicity of lung myeloid cells.
Mice were exposed intranasally with LPS or the Vehicle and 48 hours later, the cells from the lungs were harvested for analysis as described in Methods. There were 15 mice per group. Individual dots represent a pooled sample of 3 lungs; n=5 per group, and the experiment was carried out once. A. t-SNEs expression of CD11b and CD11c. B. Representative contour flow cytometry plots of cells expressing CCR1, CCR3, and CCR5. C. Dot graph of the percentage of cells expressing CCR1, CCR3, and CCR5. D. Dot graph of the absolute cell count of cells expressing CCR1, CCR3, and CCR5. E. Representative pseudocolor flow cytometry plots of CXCR2+ neutrophils, CCR1+ neutrophils, and CCR1+ monocytes. F. Dot graphs of the percentage of CXCR2+ neutrophils, CCR1+ neutrophils, and CCR1+ monocytes. G. Dot graphs of the absolute cell count of CXCR2+ neutrophils, CCR1+ neutrophils, and CCR1+ monocytes. *p<0.05, **p<0.01, ***p<0.0002, ****p<0.0001.
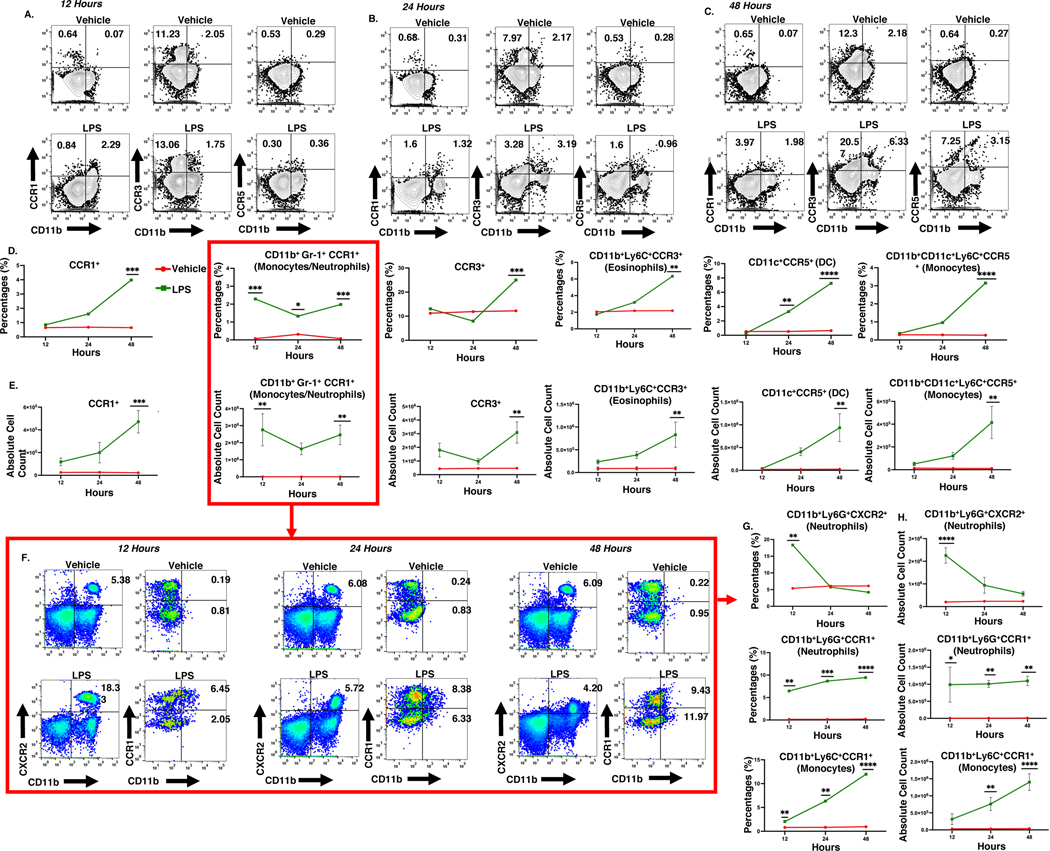
ALI alters the myeloid cells in circulation.
Heterogeneous populations of innate immune cells migrate from the bloodstream to the lungs during the ALI onset. The myeloid cell milieu migrating in circulation can change at several points during the onset phase. Therefore, we investigated the changes in myeloid cell subsets at 12, 24, and 48 hrs by immunophenotyping using flow cytometry for receptors associated with the dysregulated chemokine genes identified using lung scRNA-seq data. The data showed that LPS-challenge significantly increased the proportions of CD11b+Ly6G+CCR1+ neutrophils and CD11b+Ly6C+CCR1+ monocytes at 12, 24, and 48 hrs compared to the controls (Figure 5A–G). Flow analysis further showed that in LPS treated mice, eosinophils, and CCR5+ monocytic cell percentages and total cell count were increased at 48 hrs. We next examined the frequency of dendritic cells in circulation at several time points. Dendritic cell population percentages were increased at 24 and 48hrs after LPS exposure (Figure 5A–E). Interestingly, the CXCR2+ neutrophil population was significantly amplified in the blood of ALI mice at 12 hours. However, the levels returned to normal levels within 24 hours (Figure 5F–G). Together, these data showed that in mice with ALI, the milieu of myeloid cells in the bloodstream changes throughout the first 48 hours.
Figure 5. ALI triggers the heterogenicity of blood myeloid cells.
Mice were exposed intranasally with LPS or the Vehicle and at various time intervals, the cells from the blood were screened for various markers. There were 3 mice per group and the experiment was carried out once. A-C. Representative contour flow cytometry plots of cells expressing CCR1, CCR3, and CCR5 at 12, 24, and 48 hrs. D&E. Line graphs of the percentage of cells and absolute cell count of cells expressing CCR1, CCR3, and CCR5 at 12, 24, and 48 hrs. F. Representative pseudocolor flow cytometry plots of CXCR2+ neutrophils, CCR1+ neutrophils, and CCR1+ monocytes at 12, 24, and 48 hrs. G&H. Line graphs of CXCR2+ neutrophils, CCR1+ neutrophils, and CCR1+ monocytes cell percentage and absolute cell count at 12, 24, and 48 hrs. *p<0.05, **p<0.01, ***p<0.0002, ****p<0.0001.
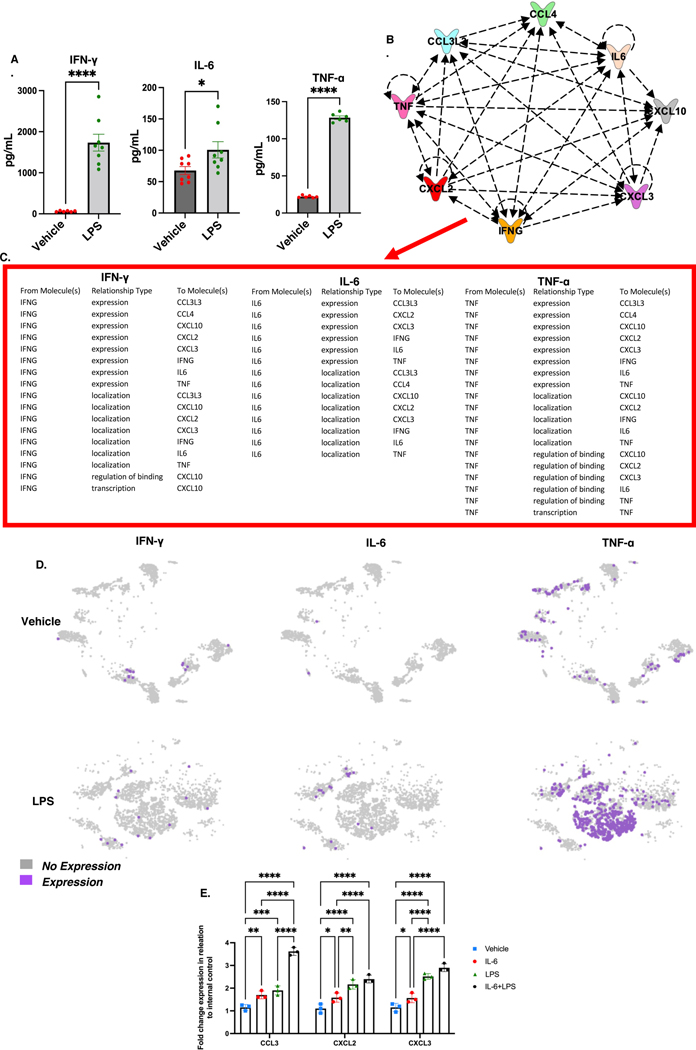
LPS-induced lung injury increases the proinflammatory cytokines in the BALF linked to migrating myeloid cells.
One of the hallmarks of Acute Respiratory Distress Syndrome (ARDS)-induced by COVID-19 and LPS is a cytokine storm consisting of excessive production of proinflammatory mediators causing lethal cytokine shock (17–19). Interestingly, in mice, the cytokine shock and lung tissue damage during ALI are associated with the induction of TNF-α, IFN-γ, and IL-6. To that end, we performed ELISAs on bronchoalveolar lavage fluid (BALF) and used IPA to generate relationship predictions. LPS-exposure increased the levels of proinflammatory cytokines INF-γ, IL-6, and TNF-α (Figure 6A). Next, we used IPA to generate pathway relationship predictions between INF-γ, IL-6, and TNF-α and chemokines CCL3/4 and CXCL2/3/10. Interestingly, INF-γ was predicted to alter the expression of CCL3/4 and CXCL2/3/10, affect the localization of CCL3 and CXCL2/3/10, and regulate the binding and transcription activity of CXCL10. On the other hand, IL-6 was predicted to associate with the regulation of the expression of CCL3/4 and CXCL3 and the localization of CCL3/4 AND CXCL2/3/10. TNF-α was predicted to alter the expression of CCL3/4 and CXCL2/3/10, affect the localization of CXCL2/10, and regulate the binding of CXCL2/3/10 (Figure 6B&C). Lastly, in scRNA-seq t-SNEs, TNF-α transcript was highly expressed in myeloid cell clusters of mice treated with LPS while the increase in the expression of INF- γ and IL-6 genes was modest (Figure 6D). However, it should be noted that the total numbers of proinflammatory macrophages and neutrophils in the blood and lungs was significantly higher in the LPS group when compared to the Vehicle control mice, which may explain why in ELISA analysis we found increased IL-6 and INF-γ protein levels in the BALF (Figure 1A&B; Figure 4A). Overall, these findings suggested relationships between INF-γ, IL-6, and TNF-α and chemotactic genes.
Figure 6. BALF protein levels of proinflammatory cytokines associated with dysregulated chemokines increased following LPS-induced ALI.
BALF was taken from the lungs of mice to perform ELISAs to screen for proinflammatory cytokines associated with immune cell trafficking. Each group had 8 mice (n=8), and the experiment was carried out once. A. BALF protein levels of IFN-γ, IL-6, and TNF-α. (n=8; per group). B and C. Predicted relationships using IPA pathway connection tool. D. scRNA-seq t-SNEs expression of IFN- γ, IL-6, and TNF-α in cells derived from the lungs. In panel E: Studying the role of IL-6 in the activation of chemokines. MNCs were isolated from groups of 5 naïve mice and cultured in the presence of IL-6, LPS or IL-6+LPS. Eighteen hours later, the cells were harvested and examined for the expression of CCL3, CXCL2, and CXCL3 using RT-qPCR. Mouse 18S primers were used as an internal control. *p<0.05, **p<0.01, ***p<0.0002, ****p<0.0001.
To further corroborate these findings, we tested the ability of IL-6 to regulate the expression of CCL3, CXCL2, and CXCL3 in lung MNCs. To that end, we isolated the lung MNCs from naïve mice and cultured them with IL-6 alone, LPS alone or a combination of IL-6+LPS. After 18 h, the cells were examined for the expression of CCL3, CXCL2, and CXCL3 using RT-qPCR. The data showed in Fig 6 E, IL-6 or LPS alone were able to induce significant upregulation of CCL3, CXCL2, and CXCL3 while the combination of IL-6 and LPS caused the highest level of increase in the expression of these molecules. Together, these data corroborated the in silico data that IL-6 does induce the expression of key chemokines.
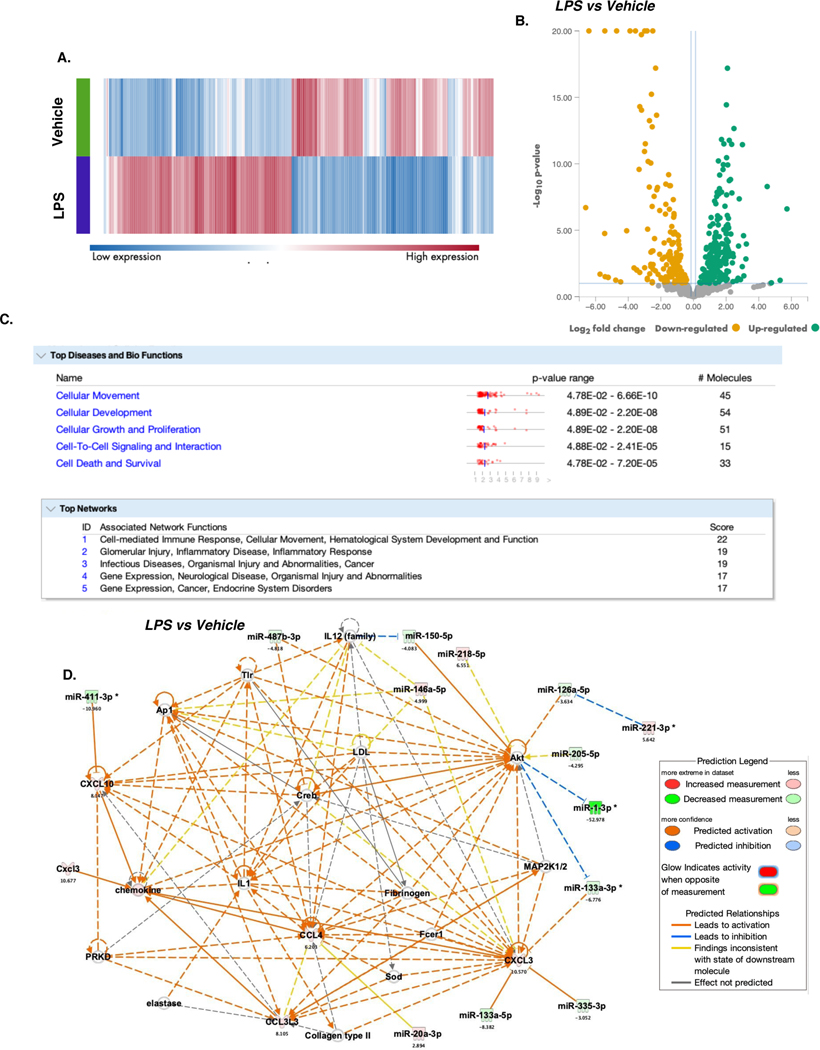
ALI-linked chemokines are downstream targets of several dysregulated miRNAs.
We next analyzed the miRNA profiles of lung cells to examine their relationships with altered chemokines expression during ALI. To explore the relationship predictions between top dysregulated genes and dysregulated miRNAs, miRNA-seq data was concatenated with scRNA-seq data and fed into IPA. QIAGEN RNA analysis portal was used to generate a heatmap of the top dysregulated miRNAs with a fold change of > 1.5 or < −1.5 (Figure 7A). The portal was also used to generate a volcano plot of the utmost significantly upregulated and downregulated miRNAs in LPS vs Veh mice (Figure 7B). Interestingly, using scRNA-seq and miRNA-seq datasets, IPA core analysis predicted dysregulated miRNAs and mRNAs dataset to be highly involved in cellular movement and cell-mediated immune response (Figure 7C). Notably, Network 1. Cell-mediated Immune Response, Cellular Movement, Hematological System, and Development and Function. Graphical summary identified connections between dysregulated chemokines and dysregulated miRNAs when LPS vs Vehicle groups were compared. In ALI animals, downregulation of miR-355–3p and miR-133a-3p/5p expression was predicted to upregulate CXCL3 expression. In addition, CXCL10 was expected to be upregulated when miR-411–3p is downregulated in ALI animals. Furthermore, IPA predicted a relationship between miR-20a-3p and CCL4. These data suggested that LPS-mediated ALI dysregulated several miRNAs which may target chemokine expression.
Figure 7. microRNA-seq analysis reveals dysregulation of several miRNAs associated with myeloid cell trafficking during ALI.
miRNA-seq was performed using the lungs from groups of 3 mice. The cells were pooled and the experiment was performed once to identify the dysregulated miRNAs associated with chemotaxis. miRNA-seq data was concatenated with scRNA-seq data and fed into IPA to predict the relationship between top dysregulated genes and dysregulated miRNAs. A. Heatmap of dysregulated miRNAs in treatment groups. B. Volcano plot of top significantly dysregulated miRNAs in Veh vs LPS mice. C. Ingenuity Pathway Analysis tool predicted the top diseases and bio-functions and associated network functions connected to dysregulated mRNAs and miRNA when comparing LPS vs Vehicle. D. Graphical summary of Network 1.
Discussion
ARDS is caused by infectious insults from a wide range of viral and bacterial pathogens. Shockingly, for 40% of patients, an ARDS diagnosis is fatal due to the limited effectiveness of current therapeutics (20–23). This number is expected to increase due to COVID-19 caused by SARS-CoV-2 virus (24), in which the patients suffering from the severe form develop ARDS (18). In such patients, the hyperimmune response and cytokine storm causes airway epithelial cell damage, making it hard to treat (18, 24–26). In addition, the increase in neutrophil frequency and abundance in the blood and respiratory tract of patients with COVID-19 and an increased abundance of neutrophils correlates to more serve forms of COVID-19 resulting in ARDS (19, 21, 27–29). Additionally, in the lungs of COVID-19 patients, there’s an enrichment of proinflammatory cytokines, chemokines and myeloid cell diversity [22, 32–37], which are also hallmarks of the LPS-induced ALI [9, 10, 13–18]. Therefore, using the LPS-induced ALI in the mouse model is significant for studying neutrophil-mediated lung injury and respiratory distress in a clinical setting. LPS-mediated lung injury is a well-recognized animal model for human ADRS (30). LPS is well known to play a critical role in sepsis because of which LPS alone has also been used in human healthy volunteers to study lung inflammation (31). However, there are limitations in the use of murine LPS model because all of the findings may not be transferable to the complexities of human ARDS although such models provide a broader understanding of the inflammatory events that occur in the lungs leading to acute lung injury.
Trafficking of myeloid cells is vital for clearing bacterial infection in the lungs during the acute phase (3, 20, 32). It’s speculated that neutrophils are the first cells to be recruited to the lungs during LPS-induced acute injury, followed by proinflammatory monocytes (10, 33). During LPS-induced ALI, residential macrophages release chemokines that bind to the chemokine receptors on neutrophils, recruiting them to the lungs. Neutrophils also release chemokines to induce the migration of pro-inflammatory monocytes to the disease site (10, 11, 34–36). As the disease progresses, hyperimmune response resulting from infiltration of myeloid cells increases, which contributes to inflammation-driven lung injury.
In the current study, we used integration of flow cytometry-based immune-profiling and sc-RNA-seq to identify changes in immune cell populations in the lungs and blood during ALI. Traditionally, flow cytometry-based immune-profiling is used to identify changes in the immune cell population during disease by phenotyping general immune cell markers, which may lead to the misclassification of immune cells expressing similar cell markers. However, scRNA-seq allows for high-resolution analysis, enabling the discovery of multiple alterations in gene expression and allows for thorough cellular characterization and identification of immune cells. Furthermore, scRNA-seq provided insights into altered chemokines ligands-receptors genes, allowing for a more focused identification of blood circulating and lung infiltrating cells during ALI. In addition, while most studies on LPS-induced ALI focus on the proinflammatory response of immune cells in the lungs during lung injury, the scope of our research was to focus on the migration of the proinflammatory myeloid cells by studying chemotaxis-associated genes. Feeding scRNA-seq data into the 10x Loupe Browser tool helped us identify the dysregulation of chemokine and chemokine receptor genes in several myeloid cell populations.
The present study demonstrated that myeloid cell populations involved during ALI are heterogeneous, regulated by the chemokine ligand-receptor axis, leading to their influx into the lungs. Under diseased conditions, there is an upshift in myeloid cells, M1 macrophages, monocytes, neutrophils, and other granulocytes, while there is a downshift in the residential alveolar macrophages, and M2 macrophages. Consistent with our findings, LPS has been shown to induce alveolar macrophage necrosis and promotion of the recruitment of neutrophils and monocytes through the chemokine ligand-receptor interactions (37). Further investigation into the dysregulation of the myeloid cell population during ALI showed that several genes coding for chemokine ligands were upregulated in myeloid cells following LPS-challenge. These gene transcripts included CCL3, CCL4, CXCL2/3, and CXCL10.
Sustained neutrophilic emigration into the alveolus contributes to lung damage (34, 38). The subset of CXCR2+ neutrophil is critical for ALI induction. CXCL2/3 are chemokines produced by monocytes and macrophages that promote the migration of neutrophils after binding to the CXCR2 expressed by the cells (35, 39–43). In LPS-induced ALI at 12 hours, CXCR2+ neutrophils rapidly increase in circulation but tither after 24 hours. The lungs from ALI mice, at 48 hours, were also found to have a substantial increase in CXCR2+ neutrophils. This suggested that CXCR2+ neutrophils migrate to the lungs during lung injury. Additionally, CCL3 and CCL4 are ligands produced by immune cells such as neutrophils, dendritic cells, and monocytes that promote CCR1+ neutrophil subset migration (3, 44–47). We noted that in LPS-challenged mice, myeloid cell populations had higher CCL3 and CCL4 expression than control groups. Interestingly, neutrophils expressing CCR1 in circulation were increased at 12, 24, and 48 hrs following LPS-challenge when compared to the control group. In addition, the levels of CCR1+ neutrophils were amplified over 12–48 hrs. The upshift of CCR1+ neutrophils was also observed in the lungs of LPS-challenged mice at 48 hrs. Interestingly, CCR1+ neutrophils contribute to cystic obstructive pulmonary disease, and blockade of CCR1 protects against acute lung injury (43, 48–51). In addition, the increased levels of CXCL10 contribute to the pathogenesis of neutrophil-mediated, excessive pulmonary inflammation during ALI. During ALI, infiltrating pulmonary neutrophils that express a unique CXCR3 receptor via TRIF was shown to be primarily responsible for increased levels of CXCL10 (52). CXCR3 is the receptor for the chemokine CXCL10 (52). In our studies, CXCL10 expression was significantly increased in neutrophil cluster 2 of ALI mice. However we did not observe a population of CXCR3 expressing cells.
In the current study, our IPA analysis to test the pathway connections between INF-γ, IL-6, and TNF-α and chemokines CCL3/4 and CXCL2/3/10 showed that INF-γ may regulate the expression of CCL3/4 and CXCL2/3/10; IL-6 on the expression of CCL3/4 and CXCL3; and TNF-α to control the expression of CCL3/4 and CXCL2/3/10 (Fig 6). In fact, our in vitro data corroborated that IL-6 does regulate the expression of CCL3, CXCL2, and CXCL3. Additionally, other studies have shown that the inflammatory chemokines CXCL9, CXCL10, and CXCL11 are predominantly induced by interferon IFN-γ (53). Also, TNF-α was found to induce CCL2 and CXCL10 (54). Together, these studies suggest that LPS-driven inflammatory cytokines may also drive some of the chemokines.
Additionally, CCL3 and CCL4 chemokines facilitate the migration of dendritic cells, monocytes, and eosinophils to the sites of lung injury. In the lungs of mice with ALI, CCL3 and CCL4 expression was upregulated in myeloid cell clusters. CCL3 and CCL4 are multifaceted chemokines that bind the CCR1 and CCR5 on monocytes and CCR3 on eosinophils (1, 3, 55–64). In circulation, the CCR1+ monocyte frequency was significantly increased at 12, 24, and 48 hrs in mice with ALI. In addition, in these mice, the levels of blood circulating CCR1+ monocytes were amplified over 12–48 hrs, while the frequency of CCR5+ monocytes was significantly increased at 48 hours. CCR1 and CCR5 are involved in the recruitment of monocytes/macrophages to the site of bacterial infection. Furthermore, CCL4 and CCL3 are secreted by neutrophils and monocytes/macrophages (44, 65, 66). CCL4 binds to CCR3, a receptor on the cell surface of eosinophils. The recruitment of eosinophils is increased in the presence of chemoattractant, CCL4 (67, 68). We noted an upshift in the CCR3+ eosinophils during LPS-mediated ALI in the circulation and in the lungs of at 48 hours.
miRNA play a key role in mediating the trafficking of immune cells during infection, including SARS-CoV-2 infection (69–71). Murata et al. 2013 reported that miR-451 downregulates neutrophils chemotaxis (72). Furthermore, miR-421 was shown to affects the chemotaxis of monocytes through CCL2 (MCP-1)(73). In the current study, we performed scRNA-seq and miRNA-seq to examine the relationship between chemotaxis associated dysregulated mRNAs when comparing LPS vs Veh groups of mice using IPA. The downregulation of miR-133a-3p/5p and miR-355–3p was predicted to upregulate CXCL3 gene expression. CXCL3 is high expressed by Neutrophils 1&2, M2/DC, M1/monocytes, and AM clusters. The downregulation of miR-411–3-3p was predicted to upregulate the expression of CXCL10. During ALI, CXCL10 was lowly expressed by Neutrophil 1–2, M1/Mono, and M2/DC during clusters. Interestingly, pulmonary observations from seriously ill patients suffering from severe inflammation due to COVID-19 had a higher frequency of inflammatory macrophages expressing cytokines/chemokines CCL2, CCL3, CCL20, CXCL1, CXCL3, CXCL10, IL8, IL1B, and TNF-α (27). Furthermore, the downregulation of miR-411–3-3p was predicted to upregulate the expression of CXCL10. During LPS-mediated ALI, CXCL10 was expressed by Neutrophil 1–2, M1/Mono, and M2/DC clusters. Interestingly, BALF taken from COVID-19 patients revealed overexpression of CCL2, CXCL10, CCL3, and CCL4 in mononuclear cells (74). In addition, CXCL10 and CCL2 protein levels in critically ill patients suffering from COVID-19 are positively correlated with fatality rates (75). Lastly, IPA analysis generated a relationship between upregulated miR-20a-3p expression and upregulation of CCL4; however, the state of the molecules was inconsistent with IPA knowledge-based prediction. CCL4 gene is primarily expressed in Neutrophil 1–3 and M2/DC clusters.
One of the limitations of this study is the use of only female mice. Previous studies have shown that the airway responsiveness of naive mice or rats to LPS is influenced by gender, with male animals exhibiting higher levels of airway inflammation when compared to females (76, 77). Also, the numbers of T cells, B cells, and macrophages present in the naive peritoneal and pleural cavities is higher in female than in male mice and rats (76). Moreover, the peritoneal macrophages from male mice express higher levels of TLR4 and produce more CXCL10 following LPS stimulation when compared to the macrophages from females (78). The heightened pro-inflammatory cytokine response of males to LPS can be reversed by the removal of androgens (79). Together, such studies have suggested that age, sex, and reproductive status may influence the immune response LPS (80). Thus, we felt that there were too many confounding factors to mix the sexes that may complicate the interpretation of the data, for which reason we used only female mice. Nonetheless, it is necessary to repeat such studies in male mice and then compare the differences between the sexes.
In summary, the current study has identified the heterogeneous population of myeloid cells that are recruited to the lungs during LPS-mediated ALI through the regulation of chemokine ligands and receptors. Interestingly, most of our flow cytometry data supported scRNA-seq data. Notably, the use of scRNA-seq analyses on cells infiltrating the lungs showed the unique nature of the chemokines and their receptors expressed on different types of immune cells, thereby providing a better characterization of immune cells while improving our understanding of how the chemokine milieu regulates the migration of myeloid cells to the lung injury sites.
Key Points:
LPS triggers upregulation of CCL3, CCL4, CXCL2/3, and CXCL10 genes.
In ALI, myeloid cells with CCR1, CCR3, CCR5, and CXCR2 receptors are recruited.
LPS regulates miRNAs that target the induction of chemokine genes.
Acknowledgements
This work was supported in part by NIH R01ES030144, R01AI123947, R01AI160896, R01AI129788, P20GM103641, and P01AT003961 to P.N. and M.N., as well as by R01 AI123947-S2 to B.L.H.
Abbreviations used:
- ARDS
Acute Respiratory Distress Syndrome
- ALI
Acute Lung Injury
- MNC
Mononuclear cells
- BALF
Bronchoalveolar lavage fluid
- IPA
Ingenuity Pathway Analysis
Footnotes
Competing interests
The authors declare that there is no conflict of interest.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Turner MD, Nedjai B, Hurst T, and Pennington DJ. 2014. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1843: 2563–2582. [DOI] [PubMed] [Google Scholar]
- 2.Muller WA 2013. Getting Leukocytes to the Site of Inflammation. Vet Pathol 50: 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokol CL, and Luster AD. 2015. The Chemokine System in Innate Immunity. Cold Spring Harb Perspect Biol 7: a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Innate (General or Nonspecific) Host Defense Mechanisms - ClinicalKey. . [Google Scholar]
- 5.Ponda MP, and Breslow JL. 2016. Serum stimulation of CCR7 chemotaxis due to coagulation factor XIIa-dependent production of high-molecular-weight kininogen domain 5. Proc Natl Acad Sci U S A 113: E7059–E7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capucetti A, Albano F, and Bonecchi R. 2020. Multiple Roles for Chemokines in Neutrophil Biology. Frontiers in Immunology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arango Duque G, and Descoteaux A. 2014. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Frontiers in Immunology 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puneet P, Moochhala S, and Bhatia M. 2005. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 288: L3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maus UA, Wellmann S, Hampl C, Kuziel WA, Srivastava M, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlöndorff D, Bohle RM, Seeger W, and Lohmeyer J. 2005. CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels. Am J Physiol Lung Cell Mol Physiol 288: L350–358. [DOI] [PubMed] [Google Scholar]
- 10.Maus UA, Waelsch K, Kuziel WA, Delbeck T, Mack M, Blackwell TS, Christman JW, Schlöndorff D, Seeger W, and Lohmeyer J. 2003. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J Immunol 170: 3273–3278. [DOI] [PubMed] [Google Scholar]
- 11.Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlöndorff D, Seeger W, and Lohmeyer J. 2002. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med 166: 268–273. [DOI] [PubMed] [Google Scholar]
- 12.Feterowski C, Mack M, Weighardt H, Bartsch B, Kaiser-Moore S, and Holzmann B. 2004. CC chemokine receptor 2 regulates leukocyte recruitment and IL-10 production during acute polymicrobial sepsis. Eur J Immunol 34: 3664–3673. [DOI] [PubMed] [Google Scholar]
- 13.Mohammed A, Alghetaa HK, Zhou J, Chatterjee S, Nagarkatti P, and Nagarkatti M. 2020. Protective effects of Δ9-tetrahydrocannabinol against enterotoxin-induced acute respiratory distress syndrome are mediated by modulation of microbiota. British Journal of Pharmacology 177: 5078–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker W, Alrafas HR, Wilson K, Miranda K, Culpepper C, Chatzistamou I, Cai G, Nagarkatti M, and Nagarkatti PS. 2020. Activation of Cannabinoid Receptor 2 Prevents Colitis-Associated Colon Cancer through Myeloid Cell De-activation Upstream of IL-22 Production. iScience 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao R, Rieder SA, Nagarkatti P, and Nagarkatti M. 2014. Staphylococcal enterotoxin B-induced microRNA-155 targets SOCS1 to promote acute inflammatory lung injury. Infect Immun 82: 2971–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holloman BL, Cannon A, Wilson K, Nagarkatti P, and Nagarkatti M. 2023. Aryl Hydrocarbon Receptor Activation Ameliorates Acute Respiratory Distress Syndrome through Regulation of Th17 and Th22 Cells in the Lungs. mBio e0313722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby R, Jonsson CB, and Kanneganti T-D. 2021. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 184: 149–168.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, and Ma X. 2020. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care 24: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, Du B, Li L-J, Zeng G, Yuen K-Y, Chen R-C, Tang C-L, Wang T, Chen P-Y, Xiang J, Li S-Y, Wang J-L, Liang Z-J, Peng Y-X, Wei L, Liu Y, Hu Y-H, Peng P, Wang J-M, Liu J-Y, Chen Z, Li G, Zheng Z-J, Qiu S-Q, Luo J, Ye C-J, Zhu S-Y, Zhong N-S, and China Medical Treatment Expert Group for Covid-19. 2020. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciesielska A, Matyjek M, and Kwiatkowska K. 2021. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 78: 1233–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami M-E, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, and Koutsoukou A. 2020. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 27: 992–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamond M, Peniston HL, Sanghavi D, and Mahapatra S. 2022. Acute Respiratory Distress Syndrome,. StatPearls Publishing. [PubMed] [Google Scholar]
- 23.Luh S, and Chiang C. 2007. Acute lung injury/acute respiratory distress syndrome (ALI/ARDS): the mechanism, present strategies and future perspectives of therapies. J Zhejiang Univ Sci B 8: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sultan M, Alghetaa H, Mohammed A, Abdulla OA, Wisniewski PJ, Singh N, Nagarkatti P, and Nagarkatti M. 2021. The Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome by Downregulating miRNA that Target Inflammatory Pathways. Front Pharmacol 12: 644281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sultan M, Wilson K, Abdulla OA, Busbee PB, Hall A, Carter T, Singh N, Chatterjee S, Nagarkatti P, and Nagarkatti M. 2021. Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis. Cells 10: 3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camporota L, Chiumello D, Busana M, Gattinoni L, and Marini JJ. 2021. Pathophysiology of COVID-19-associated acute respiratory distress syndrome. The Lancet Respiratory Medicine 9: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thürmann L, Kurth F, Völker MT, Kazmierski J, Timmermann B, Twardziok S, Schneider S, Machleidt F, Müller-Redetzky H, Maier M, Krannich A, Schmidt S, Balzer F, Liebig J, Loske J, Suttorp N, Eils J, Ishaque N, Liebert UG, von Kalle C, Hocke A, Witzenrath M, Goffinet C, Drosten C, Laudi S, Lehmann I, Conrad C, Sander L-E, and Eils R. 2020. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol 38: 970–979. [DOI] [PubMed] [Google Scholar]
- 28.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, and Zhang Z. 2020. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 26: 842–844. [DOI] [PubMed] [Google Scholar]
- 29.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, and Tian D-S. 2020. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 71: 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matute-Bello G, Frevert CW, and Martin TR. 2008. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks D, Barr LC, Wiscombe S, McAuley DF, Simpson AJ, and Rostron AJ. 2020. Human lipopolysaccharide models provide mechanistic and therapeutic insights into systemic and pulmonary inflammation. Eur Respir J 56: 1901298. [DOI] [PubMed] [Google Scholar]
- 32.Hornef MW, Wick MJ, Rhen M, and Normark S. 2002. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol 3: 1033–1040. [DOI] [PubMed] [Google Scholar]
- 33.Grommes J, and Soehnlein O. 2011. Contribution of Neutrophils to Acute Lung Injury. Mol Med 17: 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson C, and Kirsebom FCM. 2021. Neutrophils in respiratory viral infections. Mucosal Immunol 14: 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajarathnam K, Schnoor M, Richardson RM, and Rajagopal S. 2019. How do chemokines navigate neutrophils to the target site: Dissecting the structural mechanisms and signaling pathways. Cell Signal 54: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hegde VL, Singh UP, Nagarkatti PS, and Nagarkatti M. 2015. Critical Role of Mast Cells and Peroxisome Proliferator-Activated Receptor γ in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo. J. Immunol. 194: 5211–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dagvadorj J, Shimada K, Chen S, Jones HD, Tumurkhuu G, Zhang W, Wawrowsky KA, Crother TR, and Arditi M. 2015. Lipopolysaccharide Induces Alveolar Macrophage Necrosis via CD14 and the P2X7 Receptor Leading to Interleukin-1α Release. Immunity 42: 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jasper AE, McIver WJ, Sapey E, and Walton GM. 2019. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Res 8: F1000 Faculty Rev-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, and Hogg N. 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121: 4930–4937. [DOI] [PubMed] [Google Scholar]
- 40.CXCL3 - an overview | ScienceDirect Topics. . [Google Scholar]
- 41.Al-Alwan LA, Chang Y, Mogas A, Halayko AJ, Baglole CJ, Martin JG, Rousseau S, Eidelman DH, and Hamid Q. 2013. Differential Roles of CXCL2 and CXCL3 and Their Receptors in Regulating Normal and Asthmatic Airway Smooth Muscle Cell Migration. J Immunol 191: 2731–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girbl T, Lenn T, Perez L, Rolas L, Barkaway A, Thiriot A, del Fresno C, Lynam E, Hub E, Thelen M, Graham G, Alon R, Sancho D, von Andrian UH, Voisin M-B, Rot A, and Nourshargh S. 2018. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 49: 1062–1076.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metzemaekers M, Gouwy M, and Proost P. 2020. Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol 17: 433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sindhu S, Kochumon S, Shenouda S, Wilson A, Al-Mulla F, and Ahmad R. 2019. The Cooperative Induction of CCL4 in Human Monocytic Cells by TNF-α and Palmitate Requires MyD88 and Involves MAPK/NF-κB Signaling Pathways. International Journal of Molecular Sciences 20: 4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charmoy M, Brunner-Agten S, Aebischer D, Auderset F, Launois P, Milon G, Proudfoot AEI, and Tacchini-Cottier F. 2010. Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Leishmania major Inoculation in Resistant Mice. PLoS Pathog 6: e1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Repeke CE, Ferreira SB, Claudino M, Silveira EM, de Assis GF, Avila-Campos MJ, Silva JS, and Garlet GP. 2010. Evidences of the cooperative role of the chemokines CCL3, CCL4 and CCL5 and its receptors CCR1+ and CCR5+ in RANKL+ cell migration throughout experimental periodontitis in mice. Bone 46: 1122–1130. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Pang Z, Wang G, Guan X, Fang K, Wang Z, and Wang F. 2017. Advanced Role of Neutrophils in Common Respiratory Diseases. J Immunol Res 2017: 6710278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Önnervik P-O, Lindahl M, Svitacheva N, Stämpfli M, Thim K, Smailagic A, Virtala R, and Taylor JD. 2010. The role of the CCR1 receptor in the inflammatory response to tobacco smoke in a mouse model. Inflamm Res 59: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudd JM, Pulavendran S, Ashar HK, Ritchey JW, Snider TA, Malayer JR, Marie M, Chow VTK, and Narasaraju T. 2019. Neutrophils Induce a Novel Chemokine Receptors Repertoire During Influenza Pneumonia. Frontiers in Cellular and Infection Microbiology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber SN, Nowak I, Grünhage F, and Lammert F. 2021. Effects of blocking chemokine receptor CCR1 with BX471 in two models of fibrosis prevention and rescue in mice. Biochem Biophys Rep 27: 101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X, Walton W, Cook DN, Hua X, Tilley S, Haskell CA, Horuk R, Blackstock AW, and Kirby SL*. 2011. The Chemokine, CCL3, and Its Receptor, CCR1, Mediate Thoracic Radiation–Induced Pulmonary Fibrosis. Am J Respir Cell Mol Biol 45: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ichikawa A, Kuba K, Morita M, Chida S, Tezuka H, Hara H, Sasaki T, Ohteki T, Ranieri VM, dos Santos CC, Kawaoka Y, Akira S, Luster AD, Lu B, Penninger JM, Uhlig S, Slutsky AS, and Imai Y. 2013. CXCL10-CXCR3 Enhances the Development of Neutrophil-mediated Fulminant Lung Injury of Viral and Nonviral Origin. Am J Respir Crit Care Med 187: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metzemaekers M, Vanheule V, Janssens R, Struyf S, and Proost P. 2017. Overview of the Mechanisms that May Contribute to the Non-Redundant Activities of Interferon-Inducible CXC Chemokine Receptor 3 Ligands. Front Immunol 8: 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheng WS, Hu S, Ni HT, Rowen TN, Lokensgard JR, and Peterson PK. 2005. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J Leukoc Biol 78: 1233–1241. [DOI] [PubMed] [Google Scholar]
- 55.Bhatia M, Zemans RL, and Jeyaseelan S. 2012. Role of Chemokines in the Pathogenesis of Acute Lung Injury. Am J Respir Cell Mol Biol 46: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaufmann A, Salentin R, Gemsa D, and Sprenger H. 2001. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J Leukoc Biol 69: 248–252. [PubMed] [Google Scholar]
- 57.Zhao X, Gu M, Xu X, Wen X, Yang G, Li L, Sheng P, and Meng F. 2020. CCL3/CCR1 mediates CD14+CD16− circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthritis and Cartilage 28: 613–625. [DOI] [PubMed] [Google Scholar]
- 58.Dyer DP, Medina-Ruiz L, Bartolini R, Schuette F, Hughes CE, Pallas K, Vidler F, Macleod MKL, Kelly CJ, Lee KM, Hansell CAH, and Graham GJ. 2019. Chemokine Receptor Redundancy and Specificity Are Context Dependent. Immunity 50: 378–389.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruytinx P, Proost P, Van Damme J, and Struyf S. 2018. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Frontiers in Immunology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.CCL4 - an overview | ScienceDirect Topics. . [Google Scholar]
- 61.Lee D, Shin K-J, Kim DW, Yoon K-A, Choi Y-J, Lee BNR, and Cho J-Y. 2018. CCL4 enhances preosteoclast migration and its receptor CCR5 downregulation by RANKL promotes osteoclastogenesis. Cell Death Dis 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ubogu EE, Callahan MK, Tucky BH, and Ransohoff RM. 2006. CCR5 expression on monocytes and T cells: Modulation by transmigration across the blood-brain barrier in vitro. Cell Immunol 243: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zajkowska M, and Mroczko B. 2020. Eotaxins and Their Receptor in Colorectal Cancer-A Literature Review. Cancers (Basel) 12: E1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giri RK, Rajagopal V, Shahi S, Zlokovic BV, and Kalra VK. 2005. Mechanism of amyloid peptide induced CCR5 expression in monocytes and its inhibition by siRNA for Egr-1. Am J Physiol Cell Physiol 289: C264–276. [DOI] [PubMed] [Google Scholar]
- 65.Tecchio C, Micheletti A, and Cassatella MA. 2014. Neutrophil-Derived Cytokines: Facts Beyond Expression. Frontiers in Immunology 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindell DM, Standiford TJ, Mancuso P, Leshen ZJ, and Huffnagle GB. 2001. Macrophage Inflammatory Protein 1α/CCL3 Is Required for Clearance of an Acute Klebsiella pneumoniae Pulmonary Infection. Infection and Immunity 69: 6364–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shamri R, Xenakis JJ, and Spencer LA. 2011. Eosinophils in innate immunity: an evolving story. Cell Tissue Res 343: 57–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi Y, Konno Y, Kanda A, Yamada Y, Yasuba H, Sakata Y, Fukuchi M, Tomoda K, Iwai H, and Ueki S. 2019. Critical role of CCL4 in eosinophil recruitment into the airway. Clin Exp Allergy 49: 853–860. [DOI] [PubMed] [Google Scholar]
- 69.Dickey LL, Worne CL, Glover JL, Lane TE, and O’Connell RM. 2016. MicroRNA-155 enhances T cell trafficking and antiviral effector function in a model of coronavirus-induced neurologic disease. Journal of Neuroinflammation 13: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Candia P, De Rosa V, Casiraghi M, and Matarese G. 2016. Extracellular RNAs: A Secret Arm of Immune System Regulation. Journal of Biological Chemistry 291: 7221–7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guedes JR, Santana I, Cunha C, Duro D, Almeida MR, Cardoso AM, de Lima MCP, and Cardoso AL. 2016. MicroRNA deregulation and chemotaxis and phagocytosis impairment in Alzheimer’s disease. Alzheimers Dement (Amst) 3: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murata K, Yoshitomi H, Furu M, Ishikawa M, Shibuya H, Ito H, and Matsuda S. 2014. MicroRNA-451 Down-Regulates Neutrophil Chemotaxis via p38 MAPK. Arthritis & Rheumatology 66: 549–559. [DOI] [PubMed] [Google Scholar]
- 73.Zhu F, Yin J, Li J, and Xue J. 2020. MicroRNA-421 affects the chemotaxis of monocytes via MCP-1, and regulates the local immune responses in injured cartilage site of elbow joint of upper limbs. Biotechnology & Biotechnological Equipment 34: 294–302. [Google Scholar]
- 74.Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, Guo L, Yang J, Wang C, Jiang S, Yang D, Zhang G, Li H, Chen F, Xu Y, Chen M, Gao Z, Yang J, Dong J, Liu B, Zhang X, Wang W, He K, Jin Q, Li M, and Wang J. 2020. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host & Microbe 27: 883–890.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y, Wang J, Liu C, Su L, Zhang D, Fan J, Yang Y, Xiao M, Xie J, Xu Y, Li Y, and Zhang S. 2020. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Molecular Medicine 26: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, and Zeldin DC. 2006. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol 177: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kosyreva AM, Dzhalilova DS, Makarova OV, Tsvetkov IS, Zolotova NA, Diatroptova MA, Ponomarenko EA, Mkhitarov VA, Khochanskiy DN, and Mikhailova LP. 2020. Sex differences of inflammatory and immune response in pups of Wistar rats with SIRS. Sci Rep 10: 15884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marriott I, Bost KL, and Huet-Hudson YM. 2006. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol 71: 12–27. [DOI] [PubMed] [Google Scholar]
- 79.Rettew JA, Huet-Hudson YM, and Marriott I. 2008. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 78: 432–437. [DOI] [PubMed] [Google Scholar]
- 80.Klein SL, and Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol 16: 626–638. [DOI] [PubMed] [Google Scholar]