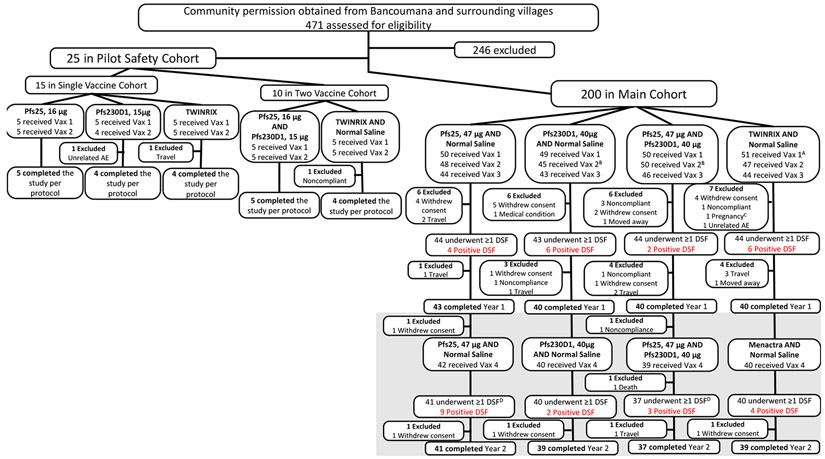
Figure 1. Trial Profile.
Trial included Pilot Safety Cohort then Main Cohort. Subjects in Pilot Safety Cohort were enrolled (April 2015) in a double-blind, comparator-controlled pilot study to receive single vaccinations (16μg Pfs25, 15μg Pfs230D1, TWINRIX) on days 0 and 28, or to receive co-administered (two syringes, separate arms) vaccinations (16μg Pfs25 + 15μg Pfs230D1, TWINRIX + normal saline) on the same schedule; pilot safety cohort participants were followed for 6 months post-dose 2 for safety and immunogenicity. For Pilot Safety Cohort, “completed study per protocol” was defined as completed through study day 196 (~6 months post vaccination #2). For analysis purposes, subjects in the TWINRIX and TWINRIX + normal saline arms (n=10) were combined as a single comparator arm. Subjects were then enrolled into the main double-blind, comparator-controlled study (n=200) and divided into 4 arms: 47μg Pfs25 + normal saline; 40μg Pfs230D1 + normal saline; 47μg Pfs25 + 40μg Pfs230D1; and comparator (TWINRIX, Menactra + normal saline). Main cohort participants were initially scheduled to receive vaccinations on a 0, 1, 6, 18-month schedule, but ultimately received these on a 0, 1, 4·5, 16·5-month schedule in order to complete dosing before the peak malaria transmission season (dose 3 = 15 Sep to 16 Oct 2015; dose 4 = 15 Sep to 17 Oct 2016). For the Main Cohort, completed Year 1= completed through study day 510 (~11 months post dose 3; end of Year 1); completed Year 2 = completed study day 730 (~6 months post dose 4). Year 2 is indicated in grey. DSF Year 1 was defined as completing at least 1 DSF from study day 175 (7 days post dose 3) to study day 213 (45 days post dose 3); maximum of 12 DSFs were completed in Year 1 (2015). DSF Year 2 was defined as completing at least 1 DSF from study day 547 (7 days post dose 4) to study day 585 (45 days post dose 4); maximum of 12 DSFs were completed in Year 2 (2016). Pfs25 = Pfs25-EPA/Alhydrogel®; Pfs230D1 = Pfs230D1-EPA/Alhydrogel®; μg = micrograms; Vax = vaccine; DSF = direct skin feeds. AOne subject randomized to 40μg Pfs230D1 + normal saline was erroneously administered comparator for vaccination #1, then continued to receive comparator throughout study (subject and clinical team remained blinded); for analysis considered comparator subject (for as-treated analysis) and Pfs230D1 subject (for ITT). BOne Pfs230D1-randomized subject was administered 47μg Pfs25 + 40μg Pfs230D1 for vaccination #2; considered Pfs230D1 subject for both as-treated and ITT analysis. COne subject (TWINRIX + normal saline) became pregnant just prior to vaccination #2 and was intentionally unblinded early for counseling of risk given vaccine received. DTwo subjects (one Pfs25; one Pfs25+Pfs230D1) did not complete a single DSF in Year 2 but completed the study.
Analysis populations:
Pilot Safety Cohort primary (safety, as-treated): Pfs25, 16μg (N=5), Pfs230D1, 15μg (N=5), TWINRIX (N=5), Pfs25, 16μg and Pfs230D1, 15μg (N=5), TWINRIX and Normal Saline (N=5)
Pilot Safety Cohort secondary (ELISA, SMFA; 2 weeks post dose 2, as-treated): Pfs25, 16μg (N=5), Pfs230D1, 15μg (N=4), TWINRIX (N=5), Pfs25, 16μg and Pfs230D1, 15μg (N=5), TWINRIX and Normal Saline (N=5)
Year 1
Main Cohort primary (safety, as-treated): Pfs25, 47μg and Normal Saline (N=50), Pfs230D1, 40μg and Normal Saline (N=49), Pfs25, 47μg and Pfs230D1, 40μg (N=50), TWINRIX and Normal Saline (N=51)
Main Cohort secondary (ELISA, SMFA; 2 weeks post dose 3, as-treated): Pfs25, 47μg and Normal Saline (N=44), Pfs230D1, 40μg and Normal Saline (N=39), Pfs25, 47μg and Pfs230D1, 40μg (N=46), TWINRIX and Normal Saline (N=44)
Main Cohort secondary (DSF, as-treated): Pfs25, 47μg and Normal Saline (N=44), Pfs230D1, 40μg and Normal Saline (N=43), Pfs25, 47μg and Pfs230D1, 40μg (N=44), TWINRIX and Normal Saline (N=44)
Year 2
Main Cohort primary (safety, as-treated): Pfs25, 47μg and Normal Saline (N=42), Pfs230D1, 40μg and Normal Saline (N=40), Pfs25, 47μg and Pfs230D1, 40μg (N=39), TWINRIX and Normal Saline (N=40)
Main Cohort secondary (ELISA, SMFA; 2 weeks post dose 4, as-treated): Pfs25, 47μg and Normal Saline (N=42), Pfs230D1, 40μg and Normal Saline (N=39), Pfs25, 47μg and Pfs230D1, 40μg (N=37), TWINRIX and Normal Saline (N=40)
Main Cohort secondary (DSF, as-treated): Pfs25, 47μg and Normal Saline (N=41), Pfs230D1, 40μg and Normal Saline (N=40), Pfs25, 47μg and Pfs230D1, 40μg (N=37), TWINRIX and Normal Saline (N=40)