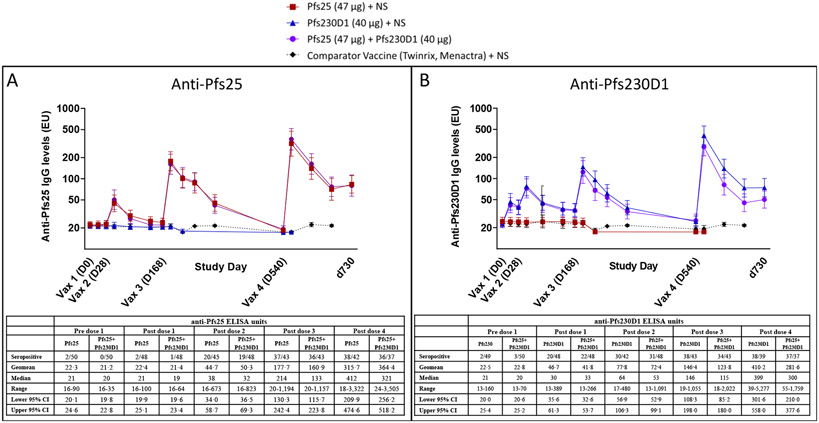
Figure 2. Antibody titres for single and combination immunogen arms by ELISA.
Anti-Main cohort participants received vaccinations on a 0, 1, 4·5, 16·5-month schedule from May to Oct 2015 (for dose 1, 2, 3) and Sep to Oct 2016 (for dose 4), and samples were collected at post-vaccination timepoints (schedule in Table S4B, Appendix, page 45) to assess anti-Pfs25 (Figure 2A) and anti-Pfs230D1 (Figure 2B) antibody titres by ELISA. Values are presented as ELISA EU. Geometric means are presented with error bars indicating 95% confidence interval. Differences in antibody titres induced by vaccines versus comparator were analyzed by Mann-Whitney test. For anti-Pfs25 titres, significant differences were observed 2 weeks after vaccinations 2, 3, and 4 (p<0·0001 for each Pfs25-containing group versus comparator). For anti-Pfs230D1 titres, significant differences were observed 2 weeks post-vaccination 1 (p=0·0001 for Pfs230D1 alone; p=0·0024 for Pfs25+Pfs230D1), and 2 weeks after each subsequent vaccination (p<0·0001 for each Pfs230D1-containing group). d730 = study day 730, ~6 months post dose 4 (end of study); comparator antibody titres to Pfs25 and Pfs230D1 were not completed for d730. NS = normal saline. Associated tables with anti-Pfs25 (Figure 2A) and anti-Pfs230D1 (Figure 2B) ELISA data at peak timepoints (2 weeks post vaccination) are provided below each associated figure. Seropositive is defined as EU > mean + 3SD of plate level of detection (Pfs25=55 EU; Pfs230D1=43 EU). Post vaccination receipt sample missingness (due to missed visit, off study post vaccination) ranged from 0-3 participants per each time point and was evenly disbursed between arms (Pfs25: pre dose 1 = 0, post dose 1 = 2 off study; post dose 2 = 3 missed visits; post dose 3 = 1 off study; post dose 4 = 0; Pfs230D1: pre dose 1 = 0, post dose 1 = 1 off study; post dose 2 = 3 missed visits; post dose 3 = 0; post dose 4 = 1 missed visit; Pfs25+Pfs230D1: pre dose 1 = 0, post dose 1 = 1 off study, 1 missed visit; post dose 2 = 2 off study; post dose 3 = 1 off study, 2 missed visits; post dose 4 = 1 off study, 1 missed visit) . Pfs25 = 47 μg of Pfs25-EPA/Alhydrogel® + normal saline; Pfs230D1 = 40 μg of Pfs230D1-EPA/Alhydrogel® + normal saline; Pfs25+Pfs230D1 = 47 μg of Pfs25-EPA/Alhydrogel® + 40 μg of Pfs230D1-EPA/Alhydrogel®; comparator = Twinrix (dose 1-3) or Menactra (dose 4) + normal saline.