Figure 4. pRALFs compete with sRALFs for FER-CVY1/ANJ/HERK1-receptor binding.
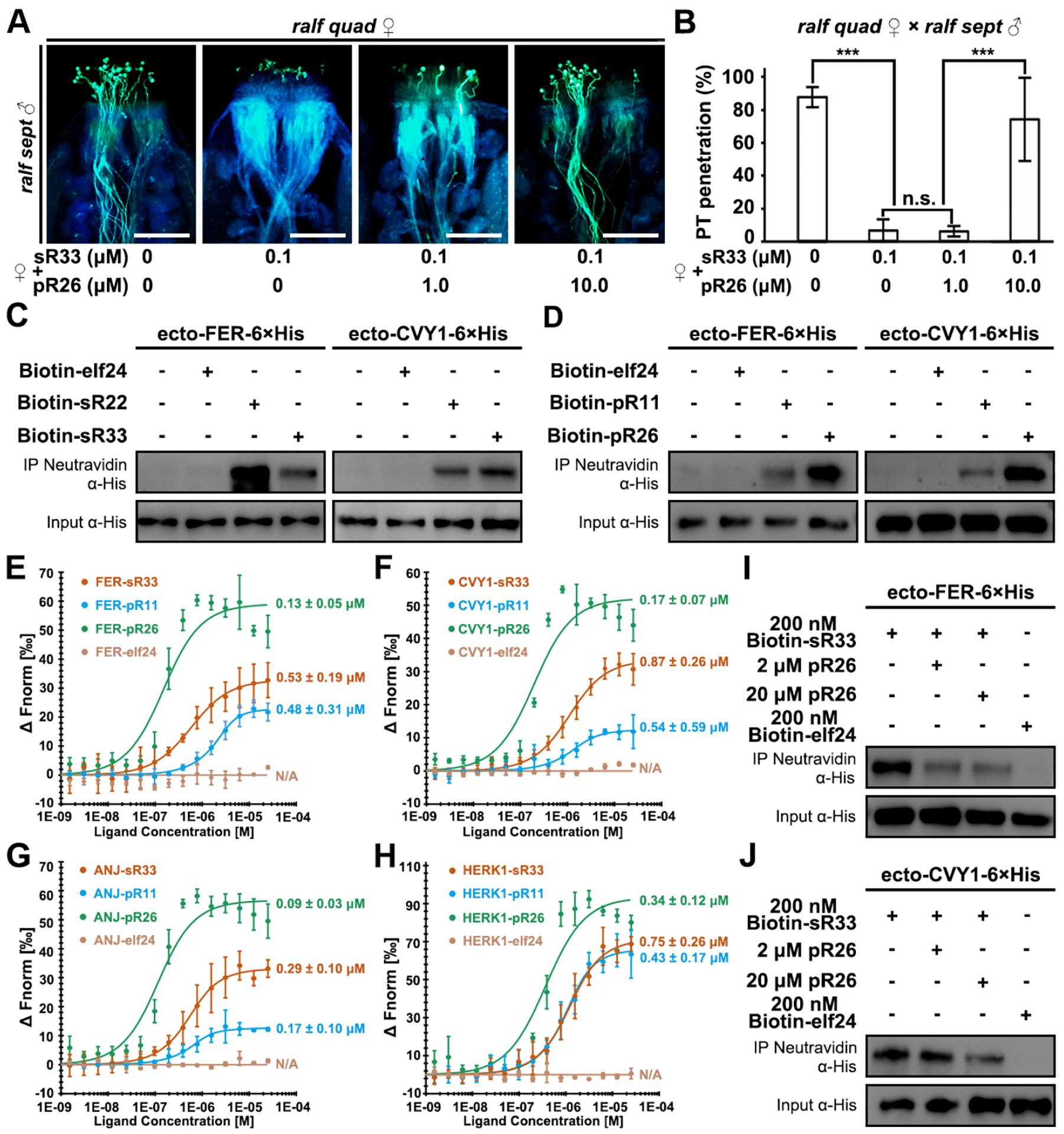
(A) Aniline blue staining showing competition results at 3 HAP. The ralf quad mutant stigmas were pre-treated with combinations of differentially concentrated sR33 and/or pR26 synthetic peptides (concentrations specified below). Scale bars, 200 μm.
(B) Statistical analysis of pollen tube penetration shown in (A).
(C) Pull-down assays showing binding activities between 6×His-tagged ectodomains of FER or CVY1 with biotinylated peptides, elf24 (negative control) and sR22/33, respectively.
(D) Pull-down assays showing binding activities between 6×His-tagged ectodomains of FER or CVY1 with biotinylated peptides, elf24 (negative control) and pR11/26, respectively.
(E-H) MST analyses show high binding affinities of sR33, pR11, and pR26, but not of elf24 (negative control) with the ectodomains of FER (E), CVY1 (F), ANJ (G), and HERK1 (H), respectively.
(I) Competitive pull-down assays showing changes in binding activities between 6×His-tagged ectodomains of FER and sR33 in the presence of an increasing amount of pR26.
(J) Competitive pull-down assays showing changes in binding activities between 6×His-tagged ectodomains of CVY and sR33 in the presence of an increasing amount of pR26.
Data in (B) are mean values ± SD; *** shows P<0.001 (Student’s t test). n.s., not significant, P>0.1 (Student’s t test). Each of the above assays was repeated at least three times.
