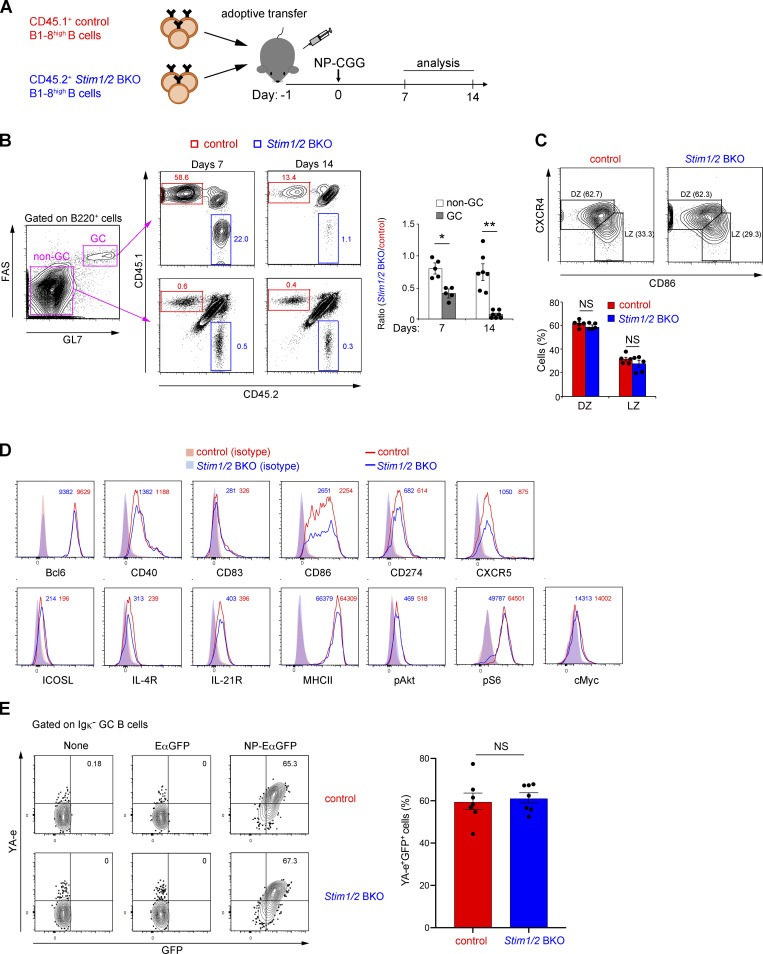
Figure 3.
GC B cell maintenance requires STIM-mediated SOC influx. (A) Schematic of experimental workflow. CD45.1/2 wild-type mice transferred with equal number of CD45.1+ control B1-8high and CD45.2+ Stim1/2 BKO B1-8high B cells were immunized with NP-CGG in alum. CD45.1/2 indicates cells from mice carrying both a CD45.1 and a CD45.2 allele. On the indicated time point (day 7 or 14), the mice were sacrificed and analyzed. (B) Flow cytometry of non-GC and GC B cells in the spleen of CD45.1/2 wild-type mice transferred with an equal number of CD45.1+ control B1-8high and CD45.2+ Stim1/2 BKO B1-8high B cells, immunized with NP-CGG in alum for 7 or 14 days. Percentages of CD45.1+ control and CD45.2+ Stim1/2 BKO cells in non-GC and GC B cells are shown. The ratios of CD45.2+ Stim1/2 BKO B cells to CD45.1+ control B cells are shown on the right. Non-GC and GC B cells are defined as FAS−GL7−B220+ and FAS+GL7+B220+ cells, respectively. *, P < 0.05, **, P < 0.001 versus non-GC B cells (Student’s t test). (C) Flow cytometry of CD45.1+ control and CD45.2+ Stim1/2 BKO GC B cells in spleen of CD45.1/2 wild-type mice transferred with equal number of CD45.1+ control B1-8high and CD45.2+ Stim1/2 BKO B1-8high B cells, immunized with NP-CGG in alum for 7 days. The percentages of DZ and LZ GC B cells are shown at the bottom. DZ and LZ GC B cells are defined as CXCR4highCD86lowCD38lowGL7+ and CXCR4lowCD86highCD38lowGL7+ cells, respectively. NS, not significant (Student’s t test). (D) Flow cytometry of CD45.1+ control (red histogram) and CD45.2+ Stim1/2 BKO GC B cells (blue histogram) in the spleen of CD45.1/2 wild-type mice transferred with equal number of CD45.1+ control B1-8high and CD45.2+ Stim1/2 BKO B1-8high B cells, immunized with NP-CGG in alum for 7 days. Red and blue shaded curves indicate isotype control staining of GC B cells. Numbers adjacent to histograms indicate mean fluorescence intensity for each marker of GC B cells. (E) Flow cytometry of CD45.1+ control and CD45.2+ Stim1/2 BKO GC B cells in spleen of CD45.1/2 wild-type mice transferred with equal number of CD45.1+ control B1-8high and CD45.2+ Stim1/2 BKO B1-8high B cells immunized with NP-CGG in alum for 7 days. Purified CD43-negative B cells from splenocytes were incubated without or with EαGFP and NP-EαGFP for 1 h before Igκ−FAS+GL7+ cells were gated and analyzed. Percentages of YA-e+GFP+ B cells are shown on the right. NS, not significant (Student’s t test). (B, C, and E) Data are presented as mean ± SEM for six or seven mice. Data shown are pooled from at least three independent experiments. (D) Data are representative of two independent experiments (n = 4).