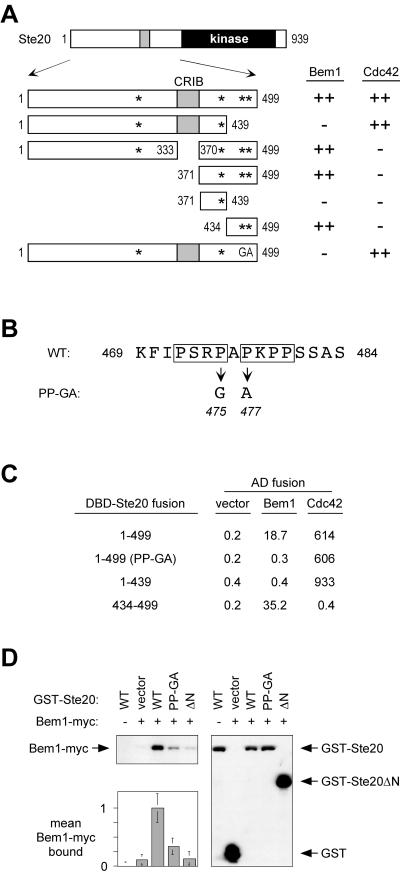
FIG. 1.
A proline-rich domain between the CRIB and kinase domains of Ste20 contains a Bem1-binding site. (A) Fragments used to map a Bem1-binding motif within Ste20 residues 434 to 499 by two-hybrid analysis. Asterisks indicate PxxP motifs; GA represents mutations in two of these motifs, as shown in panel B. DNA binding domain fusions to Ste20 fragments (from top to bottom, pB20N2, pB20N, pPP1062, pB20BA, pB20BB, pB20BC, and pPP1180) were coexpressed in PPY760 with AD fusions to Bem1157-551 (pRL51.1), Cdc42G12V/C188S (pPP1027), or the vector (pGAD424). Interactions were scored as positive (++) or negative (−), in comparison to those for vector controls, by a filter β-galactosidase assay. Bem1 interaction results were similar when full-length Bem11-551 (pRL5.2) rather than Bem1157-551 was used, though in all cases the signal was stronger with the latter. (B) Sequence showing tandem PxxP motifs (boxed) in theminimal Bem1-binding region and mutations of Pro475 and Pro477 that constitute the PP-GA allele. WT, wild type. (C) Quantitative measurements of key two-hybrid interactions from panel A, using the same strain and plasmids (pRL51.1 for Bem1). Measurements are expressed as mean β-galactosidase units (n = 3 or 4; all standard deviations were within 40% of the mean). DBD, DNA binding domain. (D) Coprecipitation of GST-Ste20 and Bem1-myc is disrupted by the PP-GA mutation. Strains expressing myc12-tagged Bem1 (+) (DLY4000) or untagged Bem1 (−) (DLY1) were transformed with a vector (pRD56) or constructs expressing the indicated Ste20 mutants (WT, PP-GA, or ΔN) as galactose-inducible GST fusions. Following galactose induction, glutathione precipitates were analyzed by immunoblotting with anti-myc (top left) or anti-GST (right) antibodies. (Bottom left) Densitometric quantification of Bem1-myc signals from four separate experiments (mean ± standard deviation), with the mean density in the negative control (GST-Ste20WT in DLY1) assigned a value of zero and that in the positive control (GST-Ste20WT in DLY4000) assigned a value of 1.