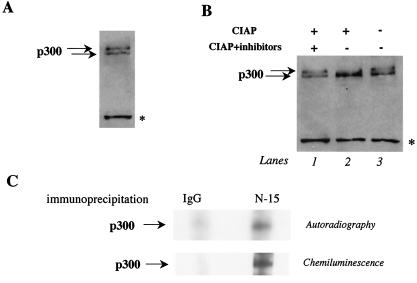
FIG. 1.
p300 phosphorylation in primary neonatal cardiomyocytes in culture. (A) p300 has slow- and fast-mobility forms in cardiomyocytes. Nuclear extracts were prepared from control cardiomyocytes maintained in cultured for 1 week and were analyzed by Western blotting on a 4% Tris-glycine gel. After transfer of the proteins, the membranes were incubated with anti-p300 antibody overnight at 4°C and p300 mobility was visualized by chemiluminescence. The asterisk represents nonspecific binding of the primary antibody. (B) The slower-migrating form of p300 is eliminated by CIAP treatment. Cardiac nuclear extracts were prepared from control cardiomyocytes and were incubated with CIAP in the absence or presence of phosphatase inhibitors. p300 mobility was then analyzed by Western blotting on a 4% Tris-glycine gel as described for panel A. (C) p300 is phosphorylated in cardiomyocytes. Primary neonatal cardiomyocytes were maintained in medium without phosphate and then labeled with [32P]orthophosphate. Total-cell lysates were immunoprecipitated with an anti-p300 or a control IgG antibody. The proteins were resolved on a 10% Tris-glycine gel. Phosphorylated p300 was visualized by autoradiography, and total p300 protein was visualized by chemiluminescence.