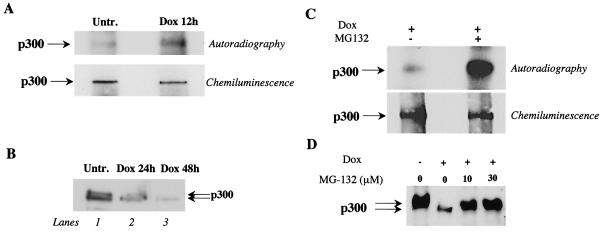
FIG. 2.
Doxorubicin induces phosphorylation of p300 in cardiomyocytes in culture, and inhibition of the proteasome activity reduces degradation of phosphorylated p300. (A) Primary cardiomyocytes were maintained in medium without phosphate and then labeled with [32P]orthophosphate with or without 1 μM doxorubicin (Dox). Total-cell lysates were prepared, and p300 was immunoprecipitated with an anti-p300 antibody. Phosphorylated p300 was visualized by autoradiography, and p300 protein was visualized by chemiluminescence. (B) Western blot analysis of nuclear extracts prepared from untreated and doxorubicin-treated primary cardiomyocytes for 24 and 48 h. (C) Primary cardiomyocytes were maintained in medium without phosphate and then labeled with [32P]orthophosphate for 15 hr in medium supplemented with 1 μM doxorubicin or in medium containing 1 μM doxorubicin plus 10 μM MG-132. Total-cell lystates were prepared, and p300 was immunoprecipitated with anti-p300 antibody. Phosphorylated p300 and total p300 were visualized as described for panel A. (D) Primary cardiocytes were treated with doxorubicin for 48 h or cotreated with doxorubicin and the proteasome inhibitor MG-132 at 10 and 30 μM. Nuclear extracts were prepared and separated on 4% Tris-glycine gel, and p300 protein was visualized with an anti-p300 antibody by cheminoluminescence.