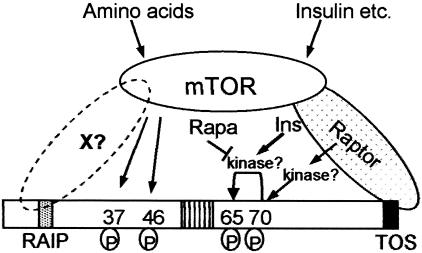
FIG. 7.
Model for regulation of the phosphorylation of 4E-BP1, integrating the findings of the present report. Our data are consistent with mTOR directly phosphorylating Thr37/46. This requires the RAIP motif in 4E-BP1, which may interact with a (putative) partner protein, X, that could also recruit mTOR to 4E-BP1. The kinase acting at Thr70 is unknown but appears unlikely to be mTOR itself, based on insensitivity to LY294002 and the lack of effect of deletion of the most C-terminal residues of mTOR. The identity of the kinase acting at Ser65 is also unknown, but this phosphorylation event is stimulated by insulin (Ins) and blocked by rapamycin (rapa). The kinase might be mTOR itself. In cells where the phosphorylation of Thr70 is low under serum-starved conditions and stimulated by insulin, this effect too is blocked by rapamycin. This suggests rapamycin may interfere with signaling from mTOR to 4E-BP1 rather than with their direct phosphorylation by mTOR. Amino acids primarily influence the phosphorylation of Thr37/46. The requirement for binding of 4E-BP1 to eIF4E for its efficient phosphorylation in vivo is not shown, for reasons of clarity.