Abstract
Rationale & Objective
Heart failure (HF) is an important cause of morbidity and mortality among individuals with chronic kidney disease (CKD). A large body of evidence from preclinical and clinical studies implicates excess levels of fibroblast growth factor 23 (FGF23) in HF pathogenesis in CKD. It remains unclear whether the relationship between elevated FGF23 levels and HF risk among individuals with CKD varies by HF subtype.
Study Design
Prospective cohort study.
Settings & Participants
A total of 3,502 participants were selected in the Chronic Renal Insufficiency Cohort study.
Exposure
Baseline plasma FGF23.
Outcomes
Incident HF by subtype and total rate of HF hospitalization. HF was categorized as HF with preserved ejection fraction (HFpEF, ejection fraction [EF] ≥ 50%), HF with reduced EF (HFrEF, EF < 50%) and HF with unknown EF (HFuEF).
Analytical Approach
Multivariable-adjusted cause-specific Cox proportional hazards models were used to investigate associations between FGF23 and incident hospitalizations for HF by subtype. The Lunn-McNeil method was used to compare hazard ratios across HF subtypes. Poisson regression models were used to evaluate the total rate of HF.
Results
During a median follow-up time of 10.8 years, 295 HFpEF, 242 HFrEF, and 156 HFuEF hospitalizations occurred. In multivariable-adjusted cause-specific Cox proportional hazards models, FGF23 was significantly associated with the incidence of HFpEF (HR, 1.41; 95% CI, 1.21-1.64), HFrEF (HR, 1.27; 95% CI, 1.05-1.53), and HFuEF (HR, 1.40; 95% CI, 1.13-1.73) per 1 standard deviation (SD) increase in the natural log of FGF23. The Lunn-McNeil method determined that the risk association was consistent across all subtypes. The rate ratio of total HF events increased with FGF23 quartile. In multivariable-adjusted models, compared with quartile 1, FGF23 quartile 4 had a rate ratio of 1.81 (95% CI, 1.28-2.57) for total HF events.
Limitations
Self-report of HF hospitalizations and possible lack of an echocardiogram at time of hospitalization.
Conclusions
In this large multicenter prospective cohort study, elevated FGF23 levels were associated with increased risks for all HF subtypes.
Plain-Language Summary
Heart failure (HF) is a prominent cause of morbidity and mortality in individuals with chronic kidney disease (CKD). Identifying potential pathways in the development of HF is essential in developing therapies to prevent and treat HF. In a large cohort of individuals with CKD, the Chronic Renal Insufficiency Cohort (N = 3,502), baseline fibroblast growth factor-23 (FGF23), a hormone that regulates phosphorous, was evaluated in relation to the development of incident and recurrent HF with reduced, preserved, and unknown ejection fraction. In this large multicenter prospective cohort study, elevated FGF23 levels were associated with increased risk of all HF subtypes. These findings demonstrate the need for further research into FGF23 as a target in preventing the development of HF in individuals with CKD.
Index Words: Fibroblast growth factor-23, heart failure, chronic renal insufficiency, left ventricular, dysfunction, phosphorous
Graphical abstract
Heart failure (HF) is a prominent comorbidity among patients with chronic kidney disease (CKD).1 According to the United States Renal Data System, >900,000 individuals with CKD have HF; population studies estimate the rate of incident HF in CKD at 18 per 1000 person-years.1,2 Hospitalizations for HF carry an alarming prognosis, with estimated 1-year mortality up to 30 percent.3 Decreased kidney function in patients with HF is associated with an increased risk of death and hospitalization.4 HF consists of HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF), each with unique prognostic implications and treatment considerations.5 Identifying pathogenic mechanisms linked to HF subtypes in patients with CKD may facilitate an individualized approach to HF prevention and management in this high-risk population.
One proposed mechanism for the development of HF in CKD is excess levels of fibroblast growth factor 23 (FGF23). FGF23 is a bone-derived hormonal regulator of phosphate and vitamin D homeostasis.6 FGF23 excess is associated with high-left ventricular mass, incident HF, atrial fibrillation, high-coronary artery calcium score, and increased mortality.7, 8, 9, 10 FGF23-induced myocardial remodeling leading to increased left ventricular mass is 1 pathway for HFpEF development.11 FGF23 excess may also contribute to HFpEF through vascular calcification12 and renal sodium handling.13 By contributing to calcitriol and Klotho reduction, FGF23 excess may indirectly promote left ventricular hypertrophy (LVH) and HFpEF.14, 15, 16
Although multiple lines of evidence support a causal role of FGF23 excess in HFpEF pathogenesis, mechanisms linking FGF23 excess with HFrEF are less well-established.17, 18, 19 Numerous potential indirect links exist between FGF23 and HFrEF. FGF23 has been linked to risk factors for ischemic heart disease that may result in cardiomyopathy and HFrEF, including systemic inflammation, vascular calcification, and HFpEF.20, 21, 22 Using data from the Chronic Renal Insufficiency Cohort (CRIC) study, we aimed to examine the specificity of associations of FGF23 excess with HF subtypes and to evaluate the rate of HF according to FGF23 levels in patients with CKD. To test the hypothesis that elevated FGF23 is associated with an increased risk of incident HFpEF but not with risk of HFrEF hospitalization, we examined the associations between baseline FGF23 level and first HF hospitalization across HF subtypes among CRIC study participants with no previous history of HF.
Methods
The CRIC Study
The CRIC study is a longitudinal prospective cohort study investigating risk factors for cardiovascular and kidney-related outcomes in patients with mild-to-moderate CKD. Between June 2003 and September 2008, 3,939 individuals aged 21-74 years with an estimated glomerular filtration rate (eGFR) of 20-70 mL/min/1.73m2 were recruited across 7 clinical centers in the United States. The CRIC study design and methods have been previously published.23 Select exclusion criteria included individuals with New York Heart Association class III or IV HF, known cirrhosis, and organ or bone marrow transplants. The study protocol was overseen by an institutional review board at each site, conducted in accordance with the Declaration of Helsinki, and participants provided written informed consent.
Study Design
To examine the association of FGF23 with the risks of incident HF subtypes and evaluate the rate of HF according to FGF23 levels, we conducted an observational study using CRIC study data.23
Study Population
Of the 3,939 participants, 60 did not have a baseline FGF23 measurement, and 377 had a self-reported history of HF on enrollment. The primary analysis included the remaining 3,502 participants (Figure S1). The primary outcome was incident HF event, sub-stratified as HFpEF, HFrEF, and HF with unknown ejection fraction (HFuEF). In secondary analyses, we studied associations of FGF23 and HF burden, defined as any HF event after enrollment, including individuals with a history of HF (N = 3,879).
Primary Exposure
Primary exposure was plasma FGF23, which was measured at baseline using a second-generation C-terminal FGF23 (cFGF23) assay (Immutopics).24 The cFGF23 assay measures both intact hormone and posttranslational C-terminal fragments. Plasma FGF23 measurements were performed in duplicate after a single thaw of frozen samples at the CRIC study central laboratory. The mean intra-assay coefficient of variation was <5% and the mean interassay coefficient of variation was 7.6%.25
Primary Outcomes
Primary outcome was time to first HF hospitalization subtype, which included HFpEF, HFrEF, and HFuEF. HF events were ascertained from baseline through May 2020 by asking participants semiannually if they were hospitalized and then obtaining hospitalization records. The first 30 discharge codes were evaluated for potential HF, and relevant records were sent for centralized review. Two or more study physicians independently reviewed HF events and deaths using history, physical examination, radiographs, and, when available, central hemodynamic monitoring and echocardiography. A HF event was defined as both reviewers agreeing on probable or definite HF based on modified clinical Framingham criteria.26 Preserved and reduced ejection fractions (EF) were defined as EF of ≥50% or ≤50%, respectively. EF was evaluated at the time of HF hospitalization. For those without an echocardiogram at the time of HF hospitalization, EF was determined by the CRIC study visit echocardiogram within 1 year before or after the HF event.27 Previous analysis demonstrated relatively stable EF over this period in CRIC participants.28,29 When no EF was available the HF hospitalization was labeled an HFuEF event. In secondary analyses, we included individuals with a history of HF before FGF23 measurement and studied associations of FGF23 with all subsequent HF events.
Finally, in sensitivity analyses, we changed our primary outcome definition for HF with preserved and reduced EF to EF of ≥40% or <40%, respectively.
Covariates
Demographic and laboratory values were collected at baseline. Participants self-reported demographics, medical history, and current medications through study questionnaires. History of cardiovascular disease (CVD) included coronary heart disease, myocardial infarction, coronary revascularization, HF, stroke, or peripheral vascular disease. Estimated GFR was calculated using the creatinine-based CKD Epidemiology Collaboration 2021 equation.30 Phosphate and total cholesterol measurements were performed at the central laboratory. Plasma parathyroid hormone (PTH) was measured using an intact scantibodies assay. The urinary protein-to-creatinine ratio was calculated using urine creatinine and urine protein and measured by a turbidometric method with benzethonium chloride. Serum iron concentration was measured by Roche (c501) autoanalyzer. Total iron-binding capacity (μG/L) was estimated as transferrin (mg/L) × 1.43, measured by immunoturbidometrics (Roche Diagnostics). Transferrin saturation was calculated as serum iron concentration (μG/L)/total iron-binding capacity (μG/L) × 100. Ferritin was measured by a sandwich immunoassay (Roche Diagnostics). High sensitivity C-reactive protein was measured by particle-enhanced immunonephelometry (Siemens).
Statistical Analysis
We summarized baseline characteristics, according to FGF23 quartiles, as means with SDs for normally distributed variables, medians with interquartile ranges for nonnormally distributed variables, and percentages for categorical variables. Cumulative incidence curves were created for each HF subtype.
To analyze the association of baseline FGF23 levels with the risk of each HF subtype, we first used separate cause-specific Cox regression models to individually analyze time to incident HF hospitalization (HFpEF, HFrEF, or HFuEF). Baseline FGF23 levels were expressed as a continuous variable with hazard ratios (HRs) calculated per SD increment of natural log-transformed (ln) FGF23 and in quartiles, with the lowest quartile defined as the reference group. In cause-specific models, participants were censored at the time of the first HF event other than the one being analyzed and otherwise followed until death, loss of follow-up, or administrative end of follow-up. To account for possible regional variability in characteristics, all adjusted models included a study site stratification term. In model 1, we adjusted for age, sex, race, and ethnicity. Model 2 was additionally adjusted for eGFR and urinary protein-to-creatinine ratio. Model 3 was additionally adjusted for potential CVD risk factors (body mass index, diabetes, smoking, systolic blood pressure, history of CVD, total cholesterol, statins, number of blood pressure medications, phosphate, and PTH). Model 4 is additionally adjusted for calcium, transferrin saturation, ferritin, and C-reactive protein. Participants without available covariates were excluded from adjusted models. Missing data was <5% for all covariates. The Kolmogorov-Smirnov test was used to verify that there was no violation of the proportionality assumption.31 In supplemental analyses, we created models as described above; however, individuals were censored at the time of kidney failure onset.
Because cause-specific Cox regression models consider one HF subtype at a time and treat time to event from another HF subtype as censored, we used the unstratified Lunn-McNeil method to simultaneously consider all cause-specific hazards in 1 model so that HRs could be compared.32 HRs were calculated per SD increment of ln FGF23. In this model, participants are considered at risk until they experience any competing outcome (HFpEF, HFrEF, and HFuEF).33 Models were adjusted for covariates, as previously mentioned. This approach allowed us to test the differential effects of FGF23 between HF subtypes, as has been previously described.34, 35, 36
In additional analyses, we included individuals with a history of HF and used adjusted Poisson regression models to evaluate total (incident and recurrent) HF hospitalization rates by FGF23 quartile.
Results
Participant Characteristics
Baseline characteristics are described in Table 1. The median FGF23 value was 138.7 (93.5-224.3) RU/mL. Individuals in the highest FGF23 quartiles were more likely to self-identify as female, Black, or Hispanic. They had higher rates of hypertension, diabetes, stroke, and CVD, lower eGFR levels, and higher PTH levels than individuals in the lower FGF23 quartiles.
Table 1.
Baseline Characteristics by FGF23 Quartile
| Total N = 3,502 | Total FGF23 N = 3,502 1.4-14,318.9 RU/mL |
FGF23 Q1 n = 876 1.4-93.5 RU/mL |
FGF23 Q2 n = 875 93.6-138.7 RU/mL |
FGF23 Q3 n = 876 138.8-224.3 RU/mL |
FGF23 Q4 n = 875 224.5-14,318.9 RU/mL |
|---|---|---|---|---|---|
| Age, y | 57.4 ± 11.2 | 55.9 ± 11.3 | 58.1 ± 10.9 | 58.1 ± 11.1 | 57.4 ± 11.2 |
| Male, n (%) | 1,925 (55.0) | 558 (63.7) | 526 (60.1) | 470 (53.7) | 371 (42.4) |
| Black, n (%) | 1,406 (40.2) | 355 (40.5) | 336 (38.4) | 333 (38.0) | 382 (43.7) |
| Hispanic, n (%) | 459 (13.1) | 64 (7.3) | 119 (13.6) | 138 (15.8) | 138 (15.8) |
| Current smoking, n (%) | 456 (13.0) | 70 (8.0) | 85 (9.7) | 120 (13.7) | 181 (20.7) |
| Body mass index, kg/m2 | 31.9 ± 7.6 | 30.4 ± 6.5 | 31.2 ± 6.9 | 32.2 ± 7.7 | 33.7 ± 8.9 |
| Systolic blood pressure, mm Hg | 128.5 ± 22.0 | 123.5 ± 19.4 | 126.8 ± 21.6 | 131.6 ± 22.7 | 132.3 ± 23.2 |
| Hypertension, n (%) | 2,997 (85.6) | 655 (74.8) | 748 (85.5) | 787 (89.8) | 807 (92.2) |
| Diabetes, n (%) | 1,618 (46.2) | 256 (29.2) | 373 (42.6) | 479 (54.7) | 510 (58.3) |
| Stroke, n (%) | 314 (9.0) | 68 (7.8) | 65 (7.4) | 84 (9.6) | 97 (11.1) |
| Cardiovascular disease, n (%) | 921 (26.3) | 166 (19.0) | 220 (25.1) | 244 (27.9) | 291 (33.3) |
| # of hypertension medications | 2.4 ± 1.3 | 1.9 ± 1.3 | 2.3 ± 1.2 | 2.5 ± 1.2 | 2.8 ± 1.1 |
| ACE/ARB use, n (%) | 2,357 (67.8) | 514 (59.2) | 611 (70.5) | 632 (72.8) | 600 (68.7) |
| eGFR, mL/min/1.73m2 | 45.0 ± 15.4 | 55.0 ± 14.4 | 48.0 ± 13.6 | 41.5 ± 12.4 | 35.5 ± 13.6 |
| 24 H Urine protein (g/24 H) | 0.18 (0.07-0.90) | 0.10 (0.06-0.32) | 0.12 (0.06-0.57) | 0.26 (0.08-1.25) | 0.46 (0.11-2.03) |
| Total cholesterol, mg/dL | 184.9 ± 45.3 | 184.0 ± 39.4 | 182.0 ± 40.5 | 186.8 ± 47.5 | 186.8 ± 52.6 |
| Statin, n (%) | 1,851 (53.3) | 385 (44.4) | 463 (53.4) | 513 (59.1) | 490 (56.1) |
| Calcium, mg/dL | 9.2 ± 0.5 | 9.2 ± 0.4 | 9.2 ± 0.5 | 9.2 ± 0.5 | 9.1 ± 0.6 |
| Phosphate, mg/dL | 3.7 ± 0.7 | 3.4 ± 0.5 | 3.6 ± 0.6 | 3.8 ± 0.6 | 4.0 ± 0.8 |
| PTH, pg/mL | 52.0 (34.0-84.7) | 39.5 (28.7-57.2) | 46.8 (32.0-72.5) | 58.8 (38.0-92.9) | 77.0 (45.9-136.3) |
| CRP, mg/L | 2.5 (1.0-6.3) | 1.8 (0.9-4.5) | 2.1 (1.0-4.9) | 2.6 (1.1-6.3) | 3.9 (1.4-8.6) |
| TSAT | 23.4 ± 8.8 | 26.0 ± 8.4 | 24.1 ± 8.1 | 23.1 ± 9.1 | 20.2 ± 8.6 |
| Ferritin, ng/mL | 155.3 (82.3-281.1) | 180.8 (100.4-325.4) | 178.8 (100.0-294.4) | 147.2 (81.6-269.7) | 119.0 (59.6-229.0) |
Note: Results are reported as mean ± standard deviation for normally distributed variables and median with interquartile range for nonnormally distributed variables.
Abbreviations: ACE/ARBs, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor 23; N, number; PTH, parathyroid hormone; Q, quartile; TSAT, transferrin saturation.
FGF23 and Incident HF
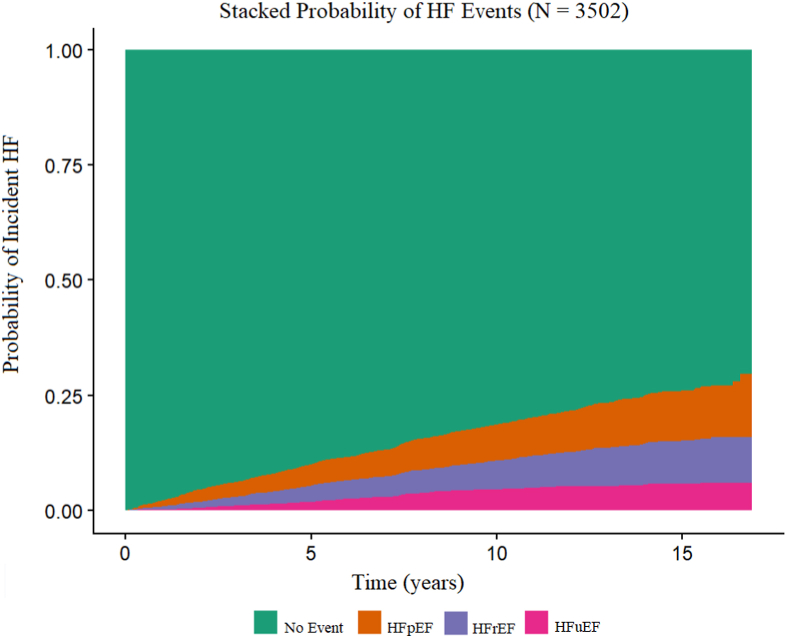
Over a median follow-up time of 10.8 years, 295 HFpEF, 242 HFrEF, and 156 HFuEF events were observed. Prehospitalization and posthospitalization echocardiography were used to classify 58 and 36 cases, respectively. The stacked probability plot of HF subtypes is displayed in Fig 1. Participants with incidents of HFuEF went on to experience all HF subtypes. Of the 156 participants who experienced an incident HFuEF event, 81 experienced a second event over the follow-up period. The second event was categorized as HFpEF in 22 participants, HFrEF in 19 participants, and HFuEF in 40 participants.
Figure 1.
Stacked Probability of Heart Failure Events. Stacked probability graph of the probability of incident heart failure event split by subtype. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HFuEF, heart failure with unknown ejection fraction.
In unadjusted cause-specific Cox proportional hazards model, higher baseline FGF23 was associated with an increased risk of incident HFpEF hospitalization (HR per SD increase in ln FGF23 1.70; 95% CI, 1.54-1.87, Table 2). FGF23 remained significantly associated with risk of incident HFpEF in multivariable-adjusted cause-specific Cox proportional hazards model (HR per SD increase in ln FGF23 1.41; 95% CI, 1.21-1.64, Table 2) and in the kidney failure-censored model (Table S1). When FGF23 was examined in quartiles, with quartile 1 as reference, in unadjusted models, there was significantly increased hazard of incident HFpEF with rising FGF23 quartile (P < 0.001). With adjustment for covariates, there was attenuation of effect in each quartile but overall P for trend remained significant (P = 0.03).
Table 2.
FGF23 and Risk of Incident Heart Failure
| FGF23 and Incident HFpEF Hazard Ratio (95%CI) |
|||||||
|---|---|---|---|---|---|---|---|
| Total N (events) | ln(FGF23) | FGF23 Q1 | FGF23 Q2 | FGF23 Q3 | FGF23 Q4 | P for Trend | |
| N, events | 3,502 (295) | 295 | 38 | 58 | 84 | 115 | |
| Unadjusted | 3,502 (295) | 1.70 (1.54-1.87) | Reference | 1.62 (1.08-2.45) | 2.66 (1.81-3.91) | 4.43 (3.07-6.41) | <0.001 |
| Model 1 | 3,502 (295) | 1.75 (1.57-1.94) | Reference | 1.47 (0.97-2.22) | 2.40 (1.63-3.55) | 4.10 (2.80-6.00) | <0.001 |
| Model 2 | 3,336 (283) | 1.49 (1.31-1.69) | Reference | 1.26 (0.83-1.93) | 1.66 (1.10-2.51) | 2.34 (1.53-3.60) | <0.001 |
| Model 3 | 3,214 (274) | 1.37 (1.19-1.58) | Reference | 1.08 (0.71-1.65) | 1.19 (0.78-1.82) | 1.54 (0.99-2.41) | 0.04 |
| Model 4 | 3,079 (264) | 1.41 (1.21-1.64) | Reference | 1.18 (0.76-1.84) | 1.31 (0.84-2.04) | 1.66 (1.03-2.66) | 0.03 |
| FGF23 and Incident HFrEF Hazard Ratio (95%CI) | |||||||
| Total N (events) | ln(FGF23) | FGF23 Q1 | FGF23 Q2 | FGF23 Q3 | FGF23 Q4 | P for trend | |
| N, events | 3,502 (242) | 242 | 38 | 49 | 73 | 82 | |
| Unadjusted | 3,502 (242) | 1.51 (1.34-1.70) | Reference | 1.39 (0.91-2.12) | 2.37 (1.60-3.51) | 3.30 (2.24-4.86) | <0.001 |
| Model 1 | 3,502 (242) | 1.64 (1.44-1.86) | Reference | 1.29 (0.84-1.98) | 2.37 (1.59-3.54) | 3.68 (2.47-5.49) | <0.001 |
| Model 2 | 3,336 (222) | 1.32 (1.13-1.55) | Reference | 1.10 (0.71-1.72) | 1.53 (0.99-2.37) | 1.97 (1.23-3.14) | 0.001 |
| Model 3 | 3,214 (210) | 1.24 (1.04-1.47) | Reference | 1.01 (0.64-1.60) | 1.27 (0.80-2.02) | 1.49 (0.90-2.47) | 0.06 |
| Model 4 | 3,079 (204) | 1.27 (1.05-1.53) | Reference | 1.03 (0.64-1.64) | 1.37 (0.85-2.21) | 1.64 (0.97-2.77) | 0.03 |
| FGF23 and Incident HFuEF Hazard Ratio (95%CI) | |||||||
| Total N (events) | ln(FGF23) | FGF23 Q1 | FGF23 Q2 | FGF23 Q3 | FGF23 Q4 | P for trend | |
| N, events | 3,502 (156) | 156 | 21 | 29 | 41 | 65 | |
| Unadjusted | 3,502 (156) | 1.67 (1.46-1.92) | Reference | 1.46 (0.84-2.57) | 2.35 (1.39-3.98) | 4.66 (2.85-7.64) | <0.001 |
| Model 1 | 3,502 (156) | 1.69 (1.45-1.97) | Reference | 1.37 (0.78-2.40) | 2.20 (1.29-3.77) | 4.32 (2.59-7.19) | <0.001 |
| Model 2 | 3,336 (148) | 1.56 (1.31-1.85) | Reference | 1.26 (0.71-2.23) | 1.68 (0.95-2.96) | 3.24 (1.83-5.73) | <0.001 |
| Model 3 | 3,214 (146) | 1.44 (1.19-1.74) | Reference | 1.11 (0.61-2.00) | 1.36 (0.76-2.44) | 2.32 (1.27-4.23) | 0.002 |
| Model 4 | 3,079 (136) | 1.40 (1.13-1.73) | Reference | 1.08 (0.59-1.99) | 1.24 (0.67-2.30) | 2.03 (1.07-3.84) | 0.02 |
Note: Risks modeled separately for each heart failure type with the use of cause-specific Cox models. Results are reported as hazard ratio per 1 standard deviation increase in natural log (ln) of fibroblast growth factor 23 (FGF23) or hazard ratio in relation to the reference quartile. Model 1: adjusted for age, sex, race, ethnicity, and study site; Model 2: Model 1 plus estimated glomerular filtration rate, and 24H urine protein; Model 3: Model 2 plus body mass index, diabetes, smoking, systolic blood pressure, any cardiovascular disease, total cholesterol, statins, number of blood pressure medications, phosphate, and parathyroid hormone; Model 4: Model 3 plus calcium, CRP (log-transformed), TSAT, and ferritin (log-transformed).
Abbreviations: HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction,; HFuEF, heart failure with unknown ejection fraction.
Similarly, in an unadjusted cause-specific Cox proportional hazards model, higher baseline FGF23 was associated with an increased risk of incident HFrEF hospitalization (HR per SD increase in ln FGF23 1.51; 95% CI, 1.34-1.70, Table 2). FGF23 remained significantly associated with risk of incident HFrEF in multivariable-adjusted cause-specific Cox proportional hazards model (HR per SD increase in ln FGF23 1.27; 95% CI, 1.05-1.53, Table 2) and in the kidney failure-censored model (Table S1). In unadjusted models with FGF23 modeled categorically and quartile 1 as reference, HR increased with rising FGF23 quartile (P < 0.001). This relationship was attenuated with multivariable adjustment (P = 0.03).
In the unadjusted cause-specific Cox proportional hazards model, higher baseline FGF23 was associated with an increased risk of incident HFuEF hospitalization (HR per SD increase in ln FGF23 1.67; 95% CI, 1.46-1.92, Table 2). FGF23 remained significantly associated with risk of incident HFuEF in multivariable-adjusted model (HR per SD increase in ln FGF23 1.40; 95% CI, 1.13-1.73, Table 2) and in the kidney failure-censored model (Table S1). With FGF23 modeled in quartiles, there was a significantly increased hazard of incident HFuEF with a rising FGF23 quartile in unadjusted models (P < 0.001). With sequential adjustment for covariates, there was an attenuation of effect in each quartile, but the overall p for trend remained significant (P = 0.02).
Results from the Lunn-McNeil method showed that the risk associations across HF subtypes were consistent, and there was no significant difference in HR of FGF23 between the 3 HF subtypes in unadjusted models (P = 0.22) or multivariable-adjusted models (P = 0.64)
In sensitivity analyses, we changed our primary outcome definition of HF with preserved and reduced EF to EF of ≥40% or <40%, respectively. The results remain qualitatively similar (Table S2).
Rate of HF Events
In additional analysis, we included 3,879 participants with baseline FGF23 measurements to study the total HF hospitalization rate associated with FGF23. In this population, there were 2,386 HF events (663 HFpEF, 870 HFrEF, and 853 HFuEF) over a median follow-up time of 10.1 years. The crude rate of HF hospitalizations was 5.49 (95% CI, 4.98-6.01) per 100 patient years (Table 3). In unadjusted models, compared with quartile 1, the rate ratio of HF events in the highest quartile of FGF23 was 4.39 (95% CI, 3.32-5.80, Table 3). This relationship remained significant in multivariable-adjusted models, with rate ratio of 1.81 (95% CI, 1.28-2.57) in FGF23 quartile 4 compared with quartile 1 (Table 3).
Table 3.
Total Heart Failure Event Rates by FGF23 Quartile
| Total N = 3,879 Events |
Overall Rate | Rate Ratio (95% CI) |
||||
|---|---|---|---|---|---|---|
| FGF23 Q1 n = 970 | FGF23 Q2 n = 970 | FGF23 Q3 n = 970 | FGF23 Q4 n =969 | |||
| N, Events | 3,879 (2,386) | 2,386 | 312 | 506 | 596 | 972 |
| Crude ratea | 3,879 (2,386) | 5.49 (4.98-6.01) | 2.52 (1.92-3.12) | 4.32 (3.35-5.29) | 5.63 (4.67-6.59) | 11.10 (9.49-12.72) |
| Unadjusted | 3879 (2,386) | 1.90 (1.72-2.11) | 1.0 (Ref) | 1.71 (1.23-2.37) | 2.22 (1.66-2.98) | 4.39 (3.32-5.80) |
| Model 1 | 3,879 (2,386) | 2.00 (1.80-2.22) | 1.0 (Ref) | 1.62 (1.17-2.25) | 2.21 (1.64-2.98) | 4.31 (3.24-5.72) |
| Model 2 | 3,695 (2,256) | 1.76 (1.55-1.99) | 1.0 (Ref) | 1.43 (1.02-2.01) | 1.61 (1.17-2.23) | 2.85 (2.05-3.96) |
| Model 3 | 3,561 (2,164) | 1.57 (1.36-1.81) | 1.0 (Ref) | 1.26 (0.89-1.78) | 1.15 (0.82-1.62) | 1.90 (1.36-2.66) |
| Model 4 | 3,404 (2,079) | 1.55 (1.33-1.80) | 1.0 (Ref) | 1.25 (0.88-1.78) | 1.12 (0.79-1.60) | 1.81 (1.28-2.57) |
Note: Model 1: adjusted for age, sex, race, ethnicity, and study site (N = 3,879); Model 2: Model 1 plus estimated glomerular filtration rate, and 24H urine protein (n = 3,695 owing to missing covariates); Model 3: Model 2 plus BMI, diabetes, smoking, systolic blood pressure, any cardiovascular disease, total cholesterol, statins, number of blood pressure medications, phosphate, and parathyroid hormone. (n = 3,561 owing to missing covariates); Model 4: Model 3 plus calcium, CRP (log-transformed), TSAT, and ferritin (log-transformed).
Abbreviation: FGF23, fibroblast growth factor 23.
Crude rates are reported as rate per 100 patient years (95% CI). Overall rates are reported as event rate per 100 patient year increase per unit increase in natural log of FGF23 (95% CI).
Discussion
In this longitudinal prospective cohort study of patients with CKD, FGF23 was independently associated with incident HF events across reduced, preserved, and unknown EF subtypes. In addition, we demonstrated a high total HF event rate in this population, greatest in those with the highest levels of FGF23. This is consistent with previous literature demonstrating associations between elevated FGF23 and incident HF.1 We did not observe significant differences in baseline FGF23 across incident HF subtypes, contrary to what has been previously reported in populations with low prevalence of CKD, in whom FGF23 was associated with incident HFpEF but not incident HFrEF.37 Our findings highlight the need for additional research into the relationships between FGF23 and HF in individuals with CKD as a possible mechanism to reduce the risk of HF.
There are many proposed mechanisms to explain the relationship between elevated FGF23 levels and HFpEF. LVH, which is highly prevalent in patients with CKD and leads to increased diastolic filling velocity and pressure, is one pathogenic mediator of HFpEF.29,38 There are several demonstrated links between elevated FGF23 levels and LVH. First, as has been shown in animal models of CKD, FGF23 activates a hypertrophic cascade in cardiac myocytes.25,39 Second, FGF23 may also have indirect effects that may promote LVH. Excess FGF23 could promote decreased renal production of Klotho, which may protect against LVH in mouse models of CKD.21,40 FGF23 may upregulate the renin-angiotensin-aldosterone system to promote LVH.41 Calcitriol may serve to inhibit the signal FGF23 exerts on cardiac myocytes, and its deficiency in CKD might contribute to LVH.42 Consistent with these possible mechanisms, we found a significant association between elevated FGF23 levels and risk of incident HFpEF in our cause-specific analysis.
Our analyses also show significant association between FGF23 and HFrEF. While data linking FGF23 and HFrEF are less ample, several possible mechanisms exist. By contributing to LVH, FGF23 could associate with HFrEF via LVH-mediated loss of cardiac contractility and HFrEF development.22 FGF23 has been associated with risk factors for ischemic heart disease. These include increased vascular calcification and systemic inflammation, known risk factors for coronary artery disease, which may lead to an increased likelihood of developing an ischemic event and subsequent HFrEF.20,43,44 In addition, elevated FGF23 has been associated with left ventricular remodeling, a pathway leading to reduced EF in post–ST-elevation myocardial infarction.45
A significant proportion of HF events in the CRIC study cohort were classified as unknown because EF was not able to be accurately characterized from the hospitalization. Previous studies demonstrate that the population characterized as HFuEF may represent a unique cohort of patients that may have worse outcomes, perhaps because of worse access to care.46,47 Despite possible unique features of the HFuEF subtype, we observed similar strengths of association between FGF23 and HFuEF as between HFpEF and HFrEF. Becasue the HFuEF subgroup is made up of those with either HFpEF or HFrEF, this result is expected and could potentially be explained by any of the previously mentioned pathologic links between elevated FGF23 and HF.
The currentg analysis differs from and expands on observations made in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort and the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study. FGF23 was uniquely associated with the incidence of HFpEF but not HFrEF in the former and incidence of HFrEF but not HFpEF in the latter.37,48 First, the use of the Lunn-McNeil method allows us to directly compare HF subtype HRs by including multiple subtype terms in one model. Despite mild differences across HF subtypes in results from individual cause-specific models, the Lunn-McNeil method demonstrated consistent associations of FGF23 with risks of HF across HFpEF, HFrEF, and HFuEF subtypes.
Second, the MESA and PREVEND study populations had a lower prevalence of CKD (eGFR < 60 mL/min/1.73m2) at baseline and less marked FGF23 elevation than observed in the CRIC study.49 A Mendelian randomization study conducted in the HF Molecular Epidemiology for Therapeutic Targets Consortium and BioVU (Vanderbilt University Medical Center biobank) datasets demonstrated that a genetically predicted FGF23 elevation was not predictive of HF in the general population. The authors found that genetically predicted FGF23 elevation was predictive of HF and HFpEF, but not HFrEF, in a population with a polygenic risk score predictive of a low eGFR.50 This compelling finding may explain in part why our CRIC study results differ from reports of studies conducted in populations with a low prevalence of CKD.
Third, instead of using imputation methods for HFuEF, as was done by the authors in the MESA study, we included HFuEF as a separate group because we noted that within the HFuEF subgroup, some individuals subsequently developed HFrEF hospitalizations, whereas others went on to have HFpEF as the second HF presentation. Finally, MESA measured intact FGF23 as opposed to C-terminal FGF23, which may account for the difference in results.
Our study had several strengths: prolonged follow-up allowed us to assess relationships between FGF23 and the development of multiple HF subtypes. HF was adjudicated by physicians who evaluated hospitalization data. We adjusted for multiple covariates and used the Lunn-McNeil method to test for differential effect of FGF23 by HF subtypes. Limitations to our approach included self-report of hospitalization by participants at follow-up visits, which may result in missed events. In addition, hospitalizations were not adjudicated for etiology of HF. In some cases, echocardiography was not obtained at the HF hospitalization, resulting in a possible misclassification of HF subtype. Finally, despite the wealth of covariate data available for our multivariable analyses, residual confounding may exist. For example, baseline intact FGF23, vitamin D stores, and Klotho were not available.
Despite current therapies, HF continues to be a significant burden for individuals with CKD. The presented analysis confirms previous data linking elevated FGF23 levels with risk of HF and extends the literature by reporting on risks with HF subtypes in a large prospective multicenter CKD cohort. Our finding that elevated FGF23 level is associated with increased risks of all HF subtypes provides further support for continued research aimed at testing elevated FGF23 as a potential therapeutic target for prevention and treatment of HF among individuals with CKD.
Article Information
CRIC Study Investigators
Lawrence J. Appel, MD, MPH, Jing Chen, MD, MMSc, MSc, Debbie L Cohen, MD, Harold I. Feldman, MD,MSCE, Alan S. Go, MD, James P. Lash, MD, Robert G. Nelson, MD, PhD, MS, Mahboob Rahman, MD, Panduranga S. Rao, MD, Vallabh O Shah, PhD, MS, Mark L. Unruh, MD, MS
Authors’ Full Names and Academic Degrees
Alexander S. Leidner, MD, Xuan Cai, MS, Leila R. Zelnick, PhD, Jungwha Lee, PhD, Nisha Bansal, MD, Andreas Pasch, MD, Mayank Kansal, MD, Jing Chen, MD, Amanda Hyre Anderson, PhD, James H. Sondheimer, MD, James P. Lash, MD, Raymond R. Townsend, MD, Alan S. Go, MD, Harold I. Feldman, MD, Sanjiv J. Shah, MD, Myles Wolf, MD, Tamara Isakova, MD, Rupal C. Mehta, MD, on behalf of the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators
Authors’ Contributions
Study Design: LZ, AP, MK, JC, AA, JS, JPL, RT, AG, HF, SS, MW; Statistical Analysis: AL, XC, LZ, NB, JL, TI, RM; Data Analysis and Interpretation: All authors contributed equally; Mentorship: SS, MW, TI, RM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Funding for the CRIC study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902 and U24DK060990). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199. Dr Leidner was supported by Ruth L. Kirschstein National Research Service Award T32 DK007169 from NIDDK. Dr Isakova was supported by grant R01DK110087 from NIDDK and grant K24HL150235 from NHLBI. Dr Shah was supported by grants U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423 from the NIH. Research presented here was in part supported by core resources from the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY) P30DK114857.
Financial Disclosure
Dr Isakova reports receiving an honorarium from Blueprint Partnership Manchester Ltd. Dr Shah reports receiving research grants from Actelion, AstraZeneca, Corvia, Novartis, and Pfizer, and consulting fees Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, GSK, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Sardocor, Shifamed, Tenax, Tenaya, and United Therapeutics. Dr Wolf has received consulting fees from Amgen, Bayer, Jnana, Reata, and Pharmacosmos, has served as a member of the Scientific Advisory Board for Unicycive and Walden, and as a member of the Board of Directors of Akebia. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received November 21, 2022. Evaluated by 1 external peer reviewer, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form July 9, 2023.
Footnotes
Complete author and article information provided before references.
Figure S1: Inclusion flow chart.
Table S1: FGF23 and Risk of Incident Heart Failure in Kidney Failure-Censored Models.
Table S2: FGF23 and Risk of Incident Heart Failure (40% EF Cutoff).
Contributor Information
Alexander S. Leidner, Email: alexander.leidner@northwestern.edu.
Chronic Renal Insufficiency Cohort (CRIC) study investigators:
Lawrence J. Appel, Jing Chen, Debbie L. Cohen, Harold I. Feldman, Alan S. Go, James P. Lash, Robert G. Nelson, Mahboob Rahman, Panduranga S. Rao, Vallabh O. Shah, and Mark L. Unruh
Supplementary Material
Figure S1; Table S1-S2.
References
- 1.Kottgen A., Russell S.D., Loehr L.R., et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18(4):1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 2.Saran R., Robinson B., Abbott K.C., et al. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 suppl 1):S1–S64. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Kociol R.D., Hammill B.G., Fonarow G.C., et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non-ADHERE Medicare beneficiaries. Am Heart J. 2010;160(5):885–892. doi: 10.1016/j.ahj.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Davis B.R., Kostis J.B., Simpson L.M., et al. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118(22):2259–2267. doi: 10.1161/circulationaha.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukumoto S., Yamashita T. FGF23 is a hormone-regulating phosphate metabolism--unique biological characteristics of FGF23. Bone. 2007;40(5):1190–1195. doi: 10.1016/j.bone.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 7.Mirza M.A., Larsson A., Melhus H., Lind L., Larsson T.E. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207(2):546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Kestenbaum B., Sachs M.C., Hoofnagle A.N., et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2014;7(3):409–417. doi: 10.1161/CIRCHEARTFAILURE.113.000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souma N., Isakova T., Lipiszko D., et al. Fibroblast growth factor 23 and cause-specific mortality in the general population: the Northern Manhattan study. J Clin Endocrinol Metab. 2016;101(10):3779–3786. doi: 10.1210/jc.2016-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta R., Cai X., Lee J., et al. Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the Chronic Renal Insufficiency Cohort study. JAMA Cardiol. 2016;1(5):548–556. doi: 10.1001/jamacardio.2016.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulus W.J., Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 12.Jimbo R., Kawakami-Mori F., Mu S., et al. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int. 2014;85(5):1103–1111. doi: 10.1038/ki.2013.332. [DOI] [PubMed] [Google Scholar]
- 13.Andrukhova O., Slavic S., Smorodchenko A., et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6(6):744–759. doi: 10.1002/emmm.201303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae S., Yalamarti B., Ke Q., et al. Preventing progression of cardiac hypertrophy and development of heart failure by paricalcitol therapy in rats. Cardiovasc Res. 2011;91(4):632–639. doi: 10.1093/cvr/cvr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J., Garami M., Cheng T., Gardner D.G. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97(7):1577–1588. doi: 10.1172/jci118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu M.C., Shi M., Cho H.J., et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2015;26(6):1290–1302. doi: 10.1681/asn.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee D.S., Gona P., Vasan R.S., et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119(24):3070–3077. doi: 10.1161/circulationaha.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaMonte M.J., FitzGerald S.J., Church T.S., et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162(5):421–429. doi: 10.1093/aje/kwi228. [DOI] [PubMed] [Google Scholar]
- 19.Munoz Mendoza J., Isakova T., Ricardo A.C., et al. Fibroblast growth factor 23 and inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. doi: 10.2215/cjn.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czaya B., Faul C. The role of fibroblast growth factor 23 in inflammation and anemia. Int J Mol Sci. 2019;20(17) doi: 10.3390/ijms20174195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J., Yoon J., An S.W., Kuro-o M., Huang C.L. Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol. 2015;26(5):1150–1160. doi: 10.1681/ASN.2014040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinzel F.R., Hohendanner F., Jin G., Sedej S., Edelmann F. Myocardial hypertrophy and its role in heart failure with preserved ejection fraction. J Appl Physiol (1985) 2015;119(10):1233–1242. doi: 10.1152/japplphysiol.00374.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman H.I., Appel L.J., Chertow G.M., et al. The chronic renal insufficiency cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14(7 suppl 2):S148–S153. doi: 10.1097/01.Asn.0000070149.78399.Ce. [DOI] [PubMed] [Google Scholar]
- 24.Isakova T., Wahl P., Vargas G.S., et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faul C., Amaral A.P., Oskouei B., et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmood S.S., Wang T.J. The epidemiology of congestive heart failure: the Framingham Heart Study perspective. Glob Hear. 2013;8(1):77–82. doi: 10.1016/j.gheart.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bansal N., Zelnick L., Bhat Z., et al. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol. 2019;73(21):2691–2700. doi: 10.1016/j.jacc.2019.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal N., Roy J., Chen H.Y., et al. Evolution of echocardiographic measures of cardiac disease from CKD to ESRD and risk of all-cause mortality: findings from the CRIC study. Am J Kidney Dis. 2018;72(3):390–399. doi: 10.1053/j.ajkd.2018.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bansal N., Keane M., Delafontaine P., et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol. 2013;8(3):355–362. doi: 10.2215/cjn.06020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karson M., Chakravarti I.M., Laha R.G., Roy J. Handbook of methods of applied statistics. J Am Stat Assoc. 1968;63(323):392–394. doi: 10.1080/01621459.1968.11009335. [DOI] [Google Scholar]
- 32.Lunn M., McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 33.Feakins B.G., McFadden E.C., Farmer A.J., Stevens R.J. Standard and competing risk analysis of the effect of albuminuria on cardiovascular and cancer mortality in patients with type 2 diabetes mellitus. Diagn Progn Res. 2018;2:13. doi: 10.1186/s41512-018-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim H.J., Zhang X., Dyck R., Osgood N. Methods of competing risks analysis of end-stage renal disease and mortality among people with diabetes. BMC Med Res Methodol. 2010;10:97. doi: 10.1186/1471-2288-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho J.E., Lyass A., Lee D.S., et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6(2):279–286. doi: 10.1161/circheartfailure.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balkan L., Ringel J.B., Levitan E.B., et al. Association of perceived stress with incident heart failure. J Card Fail. 2022;28(9):1401–1410. doi: 10.1016/j.cardfail.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almahmoud M.F., Soliman E.Z., Bertoni A.G., et al. Fibroblast growth factor-23 and heart failure with reduced versus preserved ejection fraction: MESA. J Am Heart Assoc. 2018;7(18) doi: 10.1161/JAHA.117.008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitzman D.W., Little W.C., Brubaker P.H., et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288(17):2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 39.Zile M.R., Gottdiener J.S., Hetzel S.J., et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124(23):2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 40.Kuro-o M. The klotho proteins in health and disease. Nat Rev Nephrol. 2019;15(1):27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 41.Böckmann I., Lischka J., Richter B., et al. FGF23-mediated activation of local raas promotes cardiac hypertrophy and fibrosis. Int J Mol Sci. 2019;20(18) doi: 10.3390/ijms20184634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leifheit-Nestler M., Grabner A., Hermann L., et al. Vitamin D treatment attenuates cardiac FGF23/FGFR4 signaling and hypertrophy in uremic rats. Nephrol Dial Transplant. 2017;32(9):1493–1503. doi: 10.1093/ndt/gfw454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willerson J.T., Ridker P.M. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 suppl 1):II2–I10. doi: 10.1161/01.Cir.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 44.Desjardins L., Liabeuf S., Renard C., et al. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2012;23:2017–2025. doi: 10.1007/s00198-011-1838-0. [DOI] [PubMed] [Google Scholar]
- 45.Reindl M., Reinstadler S.J., Feistritzer H.J., et al. Fibroblast growth factor 23 as novel biomarker for early risk stratification after ST-elevation myocardial infarction. Heart. 2017;103:856–862. doi: 10.1136/heartjnl-2016-310520. [DOI] [PubMed] [Google Scholar]
- 46.Muñoz M.A., Mundet-Tuduri X., Real J., et al. Heart failure labelled patients with missing ejection fraction in primary care: prognosis and determinants. BMC Fam Pract. 2017;18(1):38. doi: 10.1186/s12875-017-0612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poppe K.K., Squire I.B., Whalley G.A., et al. Known and missing left ventricular ejection fraction and survival in patients with heart failure: a MAGGIC meta-analysis report. Eur J Heart Fail. 2013;15(11):1220–1227. doi: 10.1093/eurjhf/hft101. [DOI] [PubMed] [Google Scholar]
- 48.Binnenmars S.H., Hoogslag G.E., Yeung S.M.H., et al. Fibroblast growth factor 23 and risk of new onset heart failure with preserved or reduced ejection fraction: the PREVEND study. J Am Heart Assoc. 2022;11(15) doi: 10.1161/jaha.121.024952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kestenbaum B.R., Adeney K.L., de Boer I.H., Ix J.H., Shlipak M.G., Siscovick D.S. Incidence and progression of coronary calcification in chronic kidney disease: the Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009;76(9):991–998. doi: 10.1038/ki.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akwo E., Pike M.M., Ertuglu L.A., et al. Association of genetically predicted fibroblast growth factor-23 with heart failure: a Mendelian randomization study. Clin J Am Soc Nephrol. 2022;17(8):1183–1193. doi: 10.2215/cjn.00960122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1; Table S1-S2.