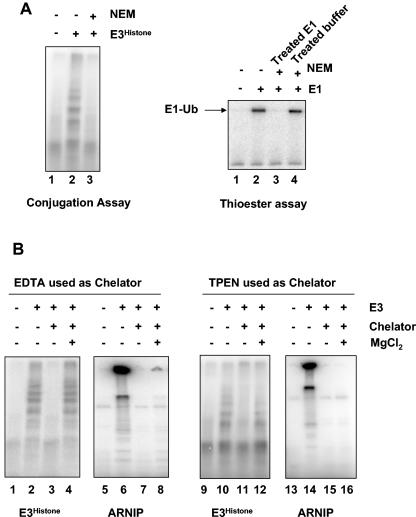
FIG. 2.
E3Histone is probably a HECT domain-containing E3. (A) E3Histone requires a free thiol group for its E3 activity. (Left panel) In the conjugation assay, E3Histone was first treated with NEM or not treated. Excess NEM was then quenched by DTT, and the other components of the conjugation assay were added. Products of the assay were resolved by SDS-PAGE and detected by autoradiography. (Right panel) To confirm that both the NEM treatment and quenching by DTT were adequate, E1 was treated sequentially with NEM and DTT (lane 3) or added subsequently to buffer that had been similarly treated with NEM and DTT (lane 4) and then assayed for the ability to form a thioester bond with 125I-labeled ubiquitin. Reaction products were resolved by SDS-PAGE at 4°C under nonreducing conditions and detected by autoradiography. (B) E3Histone does not require divalent cations for its function. E3Histone was treated with EDTA (lanes 3 and 4) or TPEN (lanes 11 and 12). Remaining chelators were then quenched with excess Mg2+ (lanes 4 and 12) or not quenched (lanes 3 and 11) prior to assays for ubiquitination of histone H2A. To confirm the efficacy of the treatment, the RING finger E3 ARNIP was similarly treated and then assayed for its autoubiquitination ability by incubation with E1, UBC4-1, and 125I-labeled ubiquitin (lanes 5 to 8 and 13 to 16). Products were resolved by SDS-PAGE and detected by autoradiography.