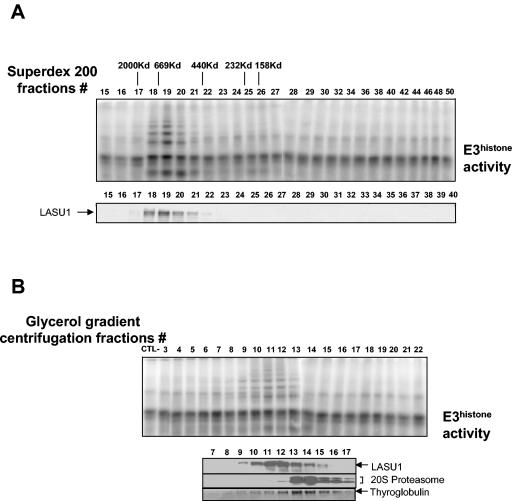
FIG. 7.
E3Histone appears to be a monomeric protein. (A) Separation of a crude testis extract using a Superdex 200 gel filtration column. (Top panel) Fractions were assayed for E3Histone activity as described in the legend to Fig. 1. Elution positions and molecular weights of the protein standards are shown. (Bottom panel) Western blot of the fractions with the anti-LASU1 antibody. The arrow shows the LASU1 band. (B) Separation of a crude testis extract by glycerol gradient centrifugation. The sample, purified 20S proteasome, or thyroglobulin was applied to a 4 to 40% glycerol gradient and subjected to the centrifugation at 30,000 rpm in an SW 40Ti rotor for 20 h at 4°C. (Top panel) Fractions from 4 to 40% glycerol gradient centrifugation were assayed for E3Histone activity. (Bottom panel) Detection of protein standards and LASU1. 20S proteasome and LASU1 were detected by Western blotting. Thyroglobulin was detected by Coomassie blue staining. (In both panels A and B, the unmodified 125I-labeled E3Histone substrate was run off the bottom of the gels.)