ABSTRACT
Background
Chronic kidney disease (CKD) patients possess a higher risk for renal cell carcinoma (RCC) possibly because of related underlying inflammation and immune dysregulation. In the current population-based cohort study, we evaluate the effects of influenza vaccination on RCC among CKD patients.
Methods
We analysed the vaccinated and unvaccinated CKD patients (≥55 years of age) identified from the Taiwan National Health Insurance Database. Propensity score matching was used to reduce the selection bias. Subgroup analyses based on comorbid conditions, dialysis status and vaccinated dosages were also conducted.
Results
The incidence of RCC decreased significantly in the vaccinated compared with unvaccinated group {unadjusted hazard ratio [HR] 0.50 [95% confidence interval (CI) 0.31–0.81], P < .01; adjusted HR 0.46 [95% CI 0.28–0.75], P < .01}. Such protective effects of influenza vaccination were noted significantly among those ≥75 years of age [unadjusted HR 0.29 (95% CI 0.12–0.74), P < .01; adjusted HR 0.22 (95% CI 0.08–0.58), P < .01]. A reverse association was noted between the total number of vaccinations and RCC events in both unadjusted and adjusted models. The Kaplan–Meier estimates of the RCC events showed significantly higher free survival rates in the vaccinated as compared with the unvaccinated patients (logrank P = .005).
Conclusion
This population-based cohort study found a significant inverse relationship between influenza vaccination and the risk of RCC in CKD patients and the protective effects were more prominent in patients >75 years of age. A possible relation exists between the total number of vaccinations and RCC events. Future randomized clinical and basic studies will be needed to prove these findings and underlying pathophysiological mechanisms.
Keywords: chronic inflammation, chronic kidney disease, immune dysfunction, influenza vaccination, renal cell carcinoma
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) is a major global public health problem and Taiwan has a relatively high incidence and prevalence of CKD and end-stage kidney disease (ESKD) compared with other countries [1, 2]. Patients with CKD are at an increased risk of renal cell carcinoma (RCC) [3]. Huang et al. [4] demonstrated that 26% of RCC patients had underlying CKD. Consistent evidence of considerable risk for RCC and other malignancies was observed among ESKD and kidney transplant recipients [5–9]. In a recent population study, Lowrance et al. [10] reported a close relationship between decreased estimated glomerular infiltration rate (eGFR) and increased risk of RCC. Studies found that such excessive risk of cancer began as early as an eGFR of 55 ml/min/1.73 m2 [11, 12] and linearly increased along with CKD deterioration. The underlying biologic mechanisms between CKD and RCC risk might be related to uraemia-induced immune dysfunction [13]. In a recent study, Rosenzweig et al. [14] revealed the association between nephrectomy type and specified metabolites with post-nephrectomy CKD status in operated RCC patients. Furthermore, CKD and RCC share common aetiologic risk factors, including tobacco abuse, hypertension, diabetes mellitus and toxins [15–20].
CKD patients had a higher risk of influenza infection [21, 22], which increased the chance of hospitalisation and cardiovascular mortality [23, 24]. In relation to observational studies, influenza vaccination reduced the risk of cardiovascular events and related morbidity and mortality among CKD/ESKD patients [25, 26]. Although not many studies are available, it was shown in animal models that Listeria monocytogenes vaccine can reduce tumour growth through antigen T-cell–specific mechanisms [27, 28]. Newman et al. [29] reported that intratumoral immune injections introduce pathogens and related components that augment the systemic antitumour immunity.
The Taiwan health authority has recognized the risk of influenza infection in CKD patients and advised an annual government-funded influenza vaccination as recommended by the Advisory Committee on Immunization Practices (ACIP) [30]. Moreover, one study showed the potential immune mechanisms involved in influenza vaccination in the inhibition of the development of human cancers [29]. As there is little information on the potential benefit of influenza vaccination in reducing RCC events in CKD patients, we conducted a population-based cohort study to determine the risk of RCC among CKD patients receiving annual influenza vaccination.
MATERIALS AND METHODS
Data source
Taiwan's National Health Insurance (NHI) program, launched in 1995, covers 99% of the population of Taiwan; currently >23 million people. The NHI Research Database (NHIRD), which is maintained by the Health and Welfare Data Science Center, has been extensively analysed and validated [31–34]. All researchers using the NHIRD and its data subsets must sign an agreement declaring that they have no intention of obtaining information that could potentially violate the privacy of patients or care providers. The current study protocol was approved by the NHIRD research committee and the Taipei Medical University Joint Institutional Review Board (N201804043).
Study cohort and study design
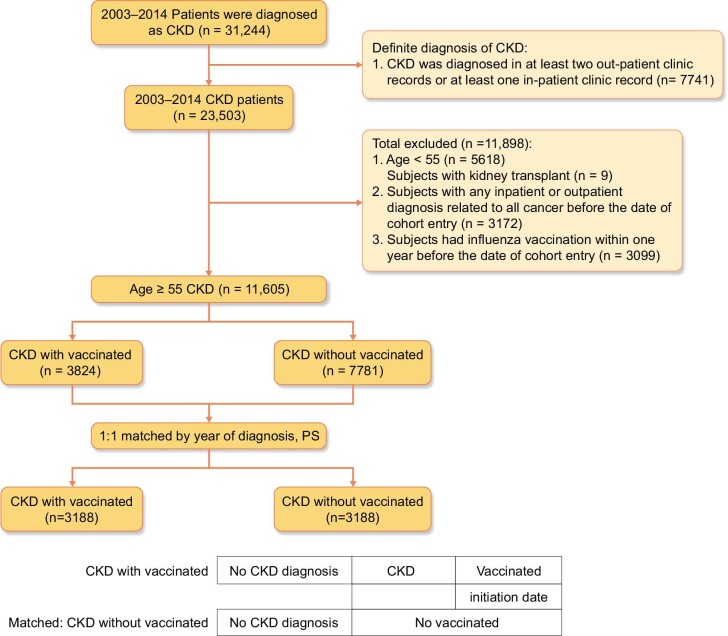
The patients enrolled in the present study were recorded as having CKD between 1 January 2003 and 31 December 2014, with all diagnoses corresponding to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 585.X. According to Taiwan's national health policy, patients ≥55 years of age with chronic diseases are recommended to receive mandatory influenza vaccination yearly without extra fees. Thus we chose those ≥55 years of age to avoid unnecessary bias. Patients ≥55 years of age (N = 11 605) with an ICD-9-CM 585.X diagnosis code were grouped as CKD only if the diagnosis code appeared at least twice during outpatient visits or once in an inpatient visit. All stages of CKD were recruited, including those undergoing renal replacement therapy. Patients with renal transplantation, subjects with any inpatient or outpatient diagnosis related to any cancers before the date of cohort enter and subjects who had influenza vaccination within 1 year before the date of cohort entry were excluded (Fig. 1). Vaccination status was identified by code V048 and/or the use of vaccine (confirmed by drug codes). After 1:1 propensity score matching and in the same year of CKD diagnosis, the patients were divided into vaccinated (n = 3188) and unvaccinated (n = 3188) groups (Fig. 1). To avoid immortal time bias [35], the vaccination date of the patients in the vaccinated group was defined as the index or cohort entry date. In the matched pairs, the participants who received and did not receive vaccination were assigned the same index date (i.e. the vaccination date) for follow-up. The study endpoint was the initial diagnosis of RCC (ICD-9-CM code 189.X). All patients were followed until RCC diagnosis, withdrawal from NHI, loss to follow-up, death or 31 December 2014. Except for those patients diagnosed as having RCC, the other data were censored.
Figure 1:
Data selection process.
Potential confounders
The potential confounders of this cohort included sociodemographic characteristics (age, sex, urbanization level and monthly income), comorbidities [coronary artery disease (CAD), heart failure (HF), peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disease, peptic ulcer, chronic liver disease, hypertension, diabetes, hyperlipidaemia, central/peripheral nervous system damage, depression], medication use [acetylsalicylic acid, statins, renin–angiotensin–aldosterone system inhibitors (RAASis) and metformin] and CKD severity (CKD-related inpatient and outpatient visits, dialysis).
Matching factors
Propensity score matching, which involves assigning levels of 0 or 1 to a treatment variable, given a set of known variables, was used to adjust for potential selection bias, confounders and differences between treatment groups in observational studies [36]. In the present study, the propensity score of each vaccinated patient was estimated by logistic regression, with the following potential confounders associated with vaccine introduction: sociodemographic characteristics (age, sex, urbanization level and monthly income), comorbidities (CAD, HF, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disease, peptic ulcer, chronic liver disease, hypertension, diabetes, hyperlipidaemia, central/peripheral nervous system damage, depression), medication use (acetylsalicylic acid, statins, RAASis and metformin) and CKD severity (CKD-related inpatient and outpatient visits, dialysis). The vaccinated and unvaccinated patients were then matched using the propensity scores and a 1:1 nearest-neighbour algorithm. As previously suggested [37], the calliper width was set as 0.03 of the pooled standard deviation of the logit of the propensity scores. Finally, the patients were divided into vaccinated (n = 3188) and unvaccinated (n = 3188) groups.
Statistical analysis
In the present study, the categorical data are expressed as numbers and percentages, while the quantitative data are presented as mean ± standard deviation (SD). The balance of characteristics was assessed by estimating the standardized differences (StDiffs) between the vaccinated and unvaccinated groups. Empirically, an absolute value of StDiffs >0.1 (10%) represents a meaningful imbalance in a given variable between two groups. A Cox proportional hazards model was used to calculate the hazard ratios (HRs) to determine the differences in the risk of RCC between the groups. The adjusted HRs were HRs that were adjusted according to the confounders. Sensitivity analysis can improve the understanding of the effects of demographic data including frequency of vaccination and comorbidity in epidemiologic database studies [38]. Thus, in the present sensitivity analysis, the patients were stratified to estimate the impact of age, sex, dialysis status, CKD-related inpatient visits, CAD, HF, peripheral vascular disease, cerebral vascular accident, peptic ulcer, chronic liver disease, hypertension, diabetes and hyperlipidaemia on the incidence of RCC with or without vaccination. The RCC-free survival rate in the vaccinated and unvaccinated patients with CKD was calculated using the Kaplan–Meier method. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). A two-tailed P-value <.05 was considered significant.
RESULTS
A total of 31 244 patients were diagnosed with CKD according to the 2003–2014 NHI database and 7741 CKD patients had a definite diagnosis of CKD according to our definition (Fig. 1). After the exclusion of patients <55 years of age (n = 5618), kidney transplant patients (n = 9), inpatient and outpatient diagnoses with any cancer (n = 3172) and those who received vaccination within 1 year before the date of cohort entry (n = 3099), 11 605 patients with CKD were included in this study (Fig. 1). A total of 3824 CKD patients received influenza vaccination and 7781 CKD patients did not receive influenza vaccination (Fig. 1). After 1:1 propensity score matching and in the same year of CKD diagnosis, the patients were divided into vaccinated (n = 3188) and unvaccinated (n = 3188) groups (Fig. 1). Table 1 shows the baseline characteristics balance before and after propensity score matching by year of diagnosis. Table 2 presents the results of propensity score matching adjusted for potential confounders defined in the method.
Table 1:
Pooled baseline characteristics balance before and after propensity score matching.
| Before matching | After matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CKD without vaccination (n = 7781) | CKD with vaccination (n = 3824) | Standardized difference | CKD without vaccination (n = 3188) | CKD with vaccination (n = 3188) | Standardized difference | |||||
| Characteristics | n | % | n | % | n | % | n | % | ||
| Propensity score, mean ± SD | 0.30 ± 0.14 | 0.39 ± 0.14 | 0.686 | 0.37 ± 0.14 | 0.38 ± 0.14 | 0.021 | ||||
| CKD-related inpatient visits, n | ||||||||||
| 0 | 5566 | 71.53 | 2592 | 67.78 | −0.082 | 2160 | 67.75 | 2188 | 68.63 | 0.019 |
| 1 | 1260 | 16.19 | 549 | 14.36 | −0.051 | 465 | 14.59 | 455 | 14.27 | −0.009 |
| ≥2 | 955 | 12.27 | 683 | 17.86 | 0.157 | 563 | 17.66 | 545 | 17.10 | −0.015 |
| Dialysis | 1807 | 23.22 | 1046 | 27.35 | 0.095 | 879 | 27.57 | 867 | 27.20 | −0.008 |
| Age (years), mean ± SD | 70.28 ± 10.38 | 72.28 ± 8.15 | 0.214 | 72.01 ± 8.82 | 72.28 ± 8.43 | 0.031 | ||||
| 55–64 | 2982 | 38.32 | 836 | 21.86 | −0.365 | 779 | 24.44 | 748 | 23.46 | −0.023 |
| 65–74 | 2182 | 28.04 | 1581 | 41.34 | 0.282 | 1206 | 37.83 | 1230 | 38.58 | 0.015 |
| ≥75 | 2617 | 33.63 | 1407 | 36.79 | 0.066 | 1203 | 37.74 | 1210 | 37.95 | 0.005 |
| Sex | ||||||||||
| Female | 3432 | 44.11 | 1677 | 43.85 | −0.005 | 1391 | 43.63 | 1405 | 44.07 | 0.009 |
| Male | 4349 | 55.89 | 2147 | 56.15 | 0.005 | 1797 | 56.37 | 1783 | 55.93 | −0.009 |
| Comorbidities | ||||||||||
| CAD | 3764 | 48.37 | 1895 | 49.56 | 0.024 | 1588 | 49.81 | 1649 | 51.73 | 0.038 |
| HF | 1877 | 24.12 | 799 | 20.89 | −0.077 | 688 | 21.58 | 721 | 22.62 | 0.025 |
| Peripheral vascular disease | 1411 | 18.13 | 604 | 15.79 | −0.062 | 503 | 15.78 | 530 | 16.62 | 0.023 |
| Cerebral vascular accident | 2774 | 35.65 | 1293 | 33.81 | −0.039 | 1087 | 34.10 | 1142 | 35.82 | 0.036 |
| Dementia | 628 | 8.07 | 272 | 7.11 | −0.036 | 244 | 7.65 | 249 | 7.81 | 0.006 |
| Pulmonary disease | 3889 | 49.98 | 1882 | 49.22 | −0.015 | 1526 | 47.87 | 1645 | 51.60 | 0.075 |
| Connective tissue disease disorder | 526 | 6.76 | 231 | 6.04 | −0.029 | 204 | 6.40 | 210 | 6.59 | 0.008 |
| Peptic ulcer | 4060 | 52.18 | 1879 | 49.14 | −0.061 | 1559 | 48.90 | 1625 | 50.97 | 0.041 |
| Chronic liver disease | 2719 | 34.94 | 1188 | 31.07 | −0.083 | 979 | 30.71 | 1032 | 32.37 | 0.036 |
| Hypertension | 6430 | 82.64 | 3117 | 81.51 | −0.029 | 2520 | 79.05 | 2580 | 80.93 | 0.047 |
| Diabetes | 4349 | 55.89 | 1882 | 49.22 | −0.134 | 1607 | 50.41 | 1634 | 51.25 | 0.017 |
| Hyperlipidaemia | 4373 | 56.20 | 1897 | 49.61 | −0.132 | 1486 | 46.61 | 1552 | 48.68 | 0.041 |
| Paraplegia | 404 | 5.19 | 155 | 4.05 | −0.054 | 135 | 4.23 | 141 | 4.42 | 0.009 |
| Depression | 453 | 5.82 | 186 | 4.86 | −0.043 | 157 | 4.92 | 171 | 5.36 | 0.020 |
| Medication use | ||||||||||
| Acetylsalicylic acid | 3004 | 38.61 | 2260 | 59.10 | 0.419 | 1777 | 55.74 | 1800 | 56.46 | 0.015 |
| Statin | 2783 | 35.77 | 1630 | 42.63 | 0.141 | 1291 | 40.50 | 1342 | 42.10 | 0.032 |
| RAASi | 4811 | 61.83 | 2949 | 77.12 | 0.337 | 2364 | 74.15 | 2410 | 75.60 | 0.033 |
| Metformin | 1603 | 20.60 | 980 | 25.63 | 0.119 | 783 | 24.56 | 802 | 25.16 | 0.014 |
| Level of urbanization | ||||||||||
| Urban | 5600 | 71.97 | 2457 | 64.25 | −0.166 | 2124 | 66.62 | 2085 | 65.40 | −0.026 |
| Suburban | 1507 | 19.37 | 875 | 22.88 | 0.086 | 705 | 22.11 | 711 | 22.30 | 0.005 |
| Rural | 674 | 8.66 | 492 | 12.87 | 0.136 | 359 | 11.26 | 392 | 12.30 | 0.032 |
| Monthly income (NT$) | ||||||||||
| 0 | 910 | 11.70 | 496 | 12.97 | 0.039 | 421 | 13.21 | 391 | 12.26 | −0.028 |
| 1–33 300 | 4807 | 61.78 | 2740 | 71.65 | 0.211 | 2214 | 69.45 | 2246 | 70.45 | 0.022 |
| ≥33 301 | 2064 | 26.53 | 588 | 15.38 | −0.277 | 553 | 17.35 | 551 | 17.28 | −0.002 |
Standardized difference: difference in the mean or proportions divided by the standard error; imbalance between groups was defined as absolute value >0.10 (corresponding to a small effect size).
Propensity score matched is adjusted for CKD-related inpatient visits, dialysis, age, sex, CAD, HF, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disorder, peptic ulcer, chronic liver disease, hypertension, diabetes, hyperlipidaemia, paraplegia, depression, acetylsalicylic acid, statin, RAASi, metformin, level of urbanization and monthly income.
Table 2:
Risk of RCC among unvaccinated and vaccinated groups in the study cohort.
| CKD without vaccination (total follow-up 8598.58 person-years ) | CKD with Vaccinated ( Total follow-up 10760.33 person-years ) | |||||
|---|---|---|---|---|---|---|
| Groups (N = 6376) | Patients with RCC, n | Incidence rate (per 105 person-years) (95% CI) | Patients with RCC, n | Incidence rate (per 105 person-years) (95% CI) | Unadjusted HR (95% CI) | Adjusted HRe (95% CI) |
| Whole cohort | 43 | 500.1 (350.6–649.6) | 26 | 241.6 (148.7–334.5) | 0.50 (0.31–0.81)** | 0.46 (0.28–0.75)** |
| Age, 55–74a | 26 | 436.5 (268.7–604.3) | 20 | 269.4 (151.4–387.5) | 0.65 (0.36–1.16) | 0.59 (0.32–1.09) |
| Age, ≥75b | 17 | 643.3 (337.5–949.1) | 6 | 179.8 (35.9–323.6) | 0.29 (0.12–0.74)** | 0.22 (0.08–0.58)** |
| Femalec | 24 | 663.6 (398.1–929.1) | 14 | 296.0 (140.9–451.0) | 0.47 (0.24–0.92)* | 0.39 (0.19–0.78)** |
| Maled | 19 | 381.4 (209.9–552.8) | 12 | 199.0 (86.4–311.6) | 0.54 (0.26–1.10) | 0.41 (0.19–0.85)* |
Total follow-up 5956.02 person-years for CKD without vaccination and 7422.86 for CKD with vaccination.
Total follow-up 2642.56 person-years for CKD without vaccination and 3337.47 for CKD with vaccination.
Total follow-up 3616.48 person-years for CKD without vaccination and 4730.20 for CKD with vaccination.
Total follow-up 4982.10 person-years for CKD without vaccination and 6030.13 for CKD with vaccination.
eMain model is adjusted for CKD-related inpatient visits, dialysis, age, sex, CAD, HF, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disorder, peptic ulcer, chronic liver disease, hypertension, diabetes, hyperlipidaemia, paraplegia, depression, acetylsalicylic acid, statin, RAASi, metformin, level of urbanization, monthly income.
The incidence of RCC was reduced significantly in the vaccinated group compared with the unvaccinated group [unadjusted HR 0.50 (95% CI 0.31–0.81), P < .01; adjusted HR 0.46 (95% CI, 0.28–0.75), P < .01]. The protective effects of the influenza vaccination were significant among those ≥75 years of age [unadjusted HR 0.29 (95% CI 0.12–0.74), P < .01; adjusted HR 0.22 (95% CI 0.08–0.58), P < .01], but not among those ages 55–74 years [unadjusted HR 0.65 (95% CI 0.36–1.16), P > .05; adjusted HR 0.59 (95% CI 0.32–1.09), P >.05]. Furthermore, the HR of RCC in the vaccinated group significantly decreased in both sexes after adjustments for modifiable risk factors, with a lower risk in females compared with males [adjusted HR 0.39 (95% CI 0.19–0.78), P < .01 in females; adjusted HR 0.41 (95% CI 0.19–0.85), P < .05 in males].
Table 3 summarizes the risks of RCC according to the total number of vaccinations in different subgroups. Both unadjusted and adjusted risk reduction of RCC was noted in those with a higher total number of vaccinations. In subgroup analysis, a significant reduction of RCC was noted among those ≥75 years of age in both sexes with influenza vaccination, regardless of dialysis status and other comorbid conditions. The Kaplan–Meier estimates of the RCC events are shown in Fig. 2. The RCC event-free survival rates in the vaccinated group were significantly higher than in the unvaccinated group (logrank P = .005). The RCC event-free survival rates increased significantly in those with a higher total number of vaccinations (Fig. 3).
Table 3:
Subgroup analysis of vaccination in risk reduction of RCC.
| Vaccinated | |||||
|---|---|---|---|---|---|
| 1 | 2–3 | ≥ 4 | |||
| Unvaccinated, adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | P for trend | |
| Unadjusted | 1.00 | 0.78 (0.39–1.56) | 0.48 (0.23–0.98)* | 0.34 (0.15–0.77)** | 0.002 |
| Model 1a | 1.00 | 0.81 (0.40–1.62) | 0.44 (0.21–0.91)* | 0.35 (0.15–0.80)* | 0.002 |
| Main modelb | 1.00 | 0.74 (0.37–1.49) | 0.37(0.18–0.78)** | 0.36 (0.16–0.82)* | 0.001 |
| Subgroup effects | |||||
| Age (years) | |||||
| 55–74 | 1.00 | 0.96 (0.42–2.19) | 0.45 (0.18–1.13) | 0.48 (0.19–1.23) | 0.046 |
| ≥75 | 1.00 | 0.22 (0.05–1.05) | 0.27 (0.08–0.94)* | 0.14 (0.02–1.10) | 0.006 |
| Sex | |||||
| Female | 1.00 | 0.67 (0.24–1.86) | 0.27 (0.10–0.75)* | 0.37 (0.12–1.17) | 0.007 |
| Male | 1.00 | 0.74 (0.26–2.08) | 0.30 (0.10–0.92)* | 0.30 (0.09–1.07) | 0.011 |
| Dialysis | |||||
| No | 1.00 | 0.41 (0.12–1.42) | 0.41 (0.14–1.21) | 0.23 (0.05–1.03) | 0.014 |
| Yes | 1.00 | 1.00 (0.41–2.45) | 0.31 (0.11–0.88)* | 0.38 (0.14–1.08) | 0.014 |
| CKD-related outpatient visits | |||||
| 0 | 1.00 | 0.27 (0.06–1.16) | 0.25 (0.07–0.86)* | 0.20 (0.04–0.86)* | 0.003 |
| 1 | 1.00 | 0.22 (0.01–6.66) | 0.58 (0.06–5.56) | 1.17 (0.05–28.78) | 0.918 |
| ≥2 | 1.00 | 1.86 (0.71–4.90) | 0.36 (0.11–1.51) | 0.46 (0.12–1.79) | 0.085 |
| CAD | |||||
| No | 1.00 | 0.87 (0.35–2.16) | 0.28 (0.10–0.82)* | 0.41 (0.15–1.09) | 0.011 |
| Yes | 1.00 | 0.57 (0.17–1.91) | 0.56 (0.19–1.65) | 0.26 (0.05–1.39) | 0.071 |
| HF | |||||
| No | 1.00 | 0.82 (0.37–1.81) | 0.39 (0.17–0.92)* | 0.44 (0.18–1.09) | 0.014 |
| Yes | 1.00 | 0.28 (0.04–1.84) | 0.06 (0.01–0.35)** | – | 0.002 |
| Peripheral vascular disease | |||||
| No | 1.00 | 0.77 (0.37–1.60) | 0.32 (0.14–0.73)** | 0.35 (0.15–0.85)* | 0.001 |
| Yes | 1.00 | 0.33 (0.01–10.72) | 0.24 (0.01–9.86) | 0.93 (0.02–39.50) | 0.697 |
| Cerebral vascular accident | |||||
| No | 1.00 | 0.93 (0.40–2.15) | 0.45 (0.19–1.04) | 0.49 (0.21–1.17) | 0.032 |
| Yes | 1.00 | 0.38 (0.09–1.60) | 0.12 (0.02–0.59)** | – | 0.006 |
| Pulmonary disease | |||||
| No | 1.00 | 0.51 (0.17–1.49) | 0.50 (0.20–1.25) | 0.35 (0.12–1.03) | 0.023 |
| Yes | 1.00 | 0.82 (0.30–2.22) | 0.19 (0.05–0.71)* | 0.37 (0.10–1.42) | 0.014 |
| Peptic ulcer | |||||
| No | 1.00 | 0.95 (0.40–2.25) | 0.39 (0.14–1.08) | 0.29 (0.09–1.01) | 0.015 |
| Yes | 1.00 | 0.44 (0.12–1.55) | 0.33 (0.11–1.03) | 0.45 (0.14–1.43) | 0.045 |
| Chronic liver disease | |||||
| No | 1.00 | 0.48 (0.18–1.28) | 0.29 (0.11–0.78)* | 0.25 (0.09–0.75)* | 0.001 |
| Yes | 1.00 | 1.22 (0.40–3.67) | 0.65 (0.20–2.07) | 0.54 (0.14–2.11) | 0.306 |
| Hypertension | |||||
| No | 1.00 | 0.40 (0.06–2.65) | 6.62 (0.92–47.77) | – | 0.024 |
| Yes | 1.00 | 0.77 (0.34–1.71) | 0.45 (0.20–1.00)* | 0.47 (0.20–1.13) | 0.023 |
| Diabetes | |||||
| No | 1.00 | 0.57 (0.20–1.64) | 0.23 (0.07–0.78)* | 0.43 (0.16–1.20) | 0.011 |
| Yes | 1.00 | 1.02 (0.37–2.81) | 0.71 (0.26–1.96) | 0.28 (0.06–1.33) | 0.115 |
| Hyperlipidaemia | |||||
| No | 1.00 | 0.49 (0.16–1.48) | 0.32 (0.09–1.06) | 0.33 (0.10–1.13) | 0.015 |
| Yes | 1.00 | 0.81 (0.30–2.21) | 0.46 (0.17–1.25) | 0.34 (0.09–1.25) | 0.047 |
*P < .05, **P < .01, ***P < .001.
aModel 1 is adjusted for CKD-related inpatient visits, dialysis, age, sex, CAD, HF, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disorder, peptic ulcer, chronic liver disease, hypertension, diabetes, hyperlipidaemia, paraplegia, depression, level of urbanization and monthly income.
bMain model is adjusted for CKD-related inpatient visits, dialysis, age, sex, CAD, HF, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disorder, peptic ulcer, chronic liver disease, hypertension, diabetes, hyperlipidaemia, paraplegia, depression, acetylsalicylic acid, statin, RAASi, metformin, level of urbanization and monthly income.
Figure 2:
RCC events-free survival rates (n = 6376) from 1 January 2003 to 31 December 2014 in Taiwan, stratified by vaccinated and unvaccinated CKD patients (logrank test, χ2 = 7.957, df = 1, P = .005).
Figure 3:
RCC events-free survival rates (n = 6376) from 1 January 2003 to 31 December 2014 in Taiwan, stratified according to the total number of vaccinations (logrank test, χ2 = 10.013, df = 3, P = .018).
DISCUSSION
Our study demonstrated that CKD patients who had received influenza vaccination exhibited a lower risk of RCC events than those who were unvaccinated in both sexes and the protective effects were more prominent in patients ≥75 years of age. Among the dialysis patients, the risk of RCC events was lower in the vaccinated than the unvaccinated group. The cumulative RCC event-free survival rates in the vaccinated group were significantly higher than in the unvaccinated group. RCC event-free survival rates were positively related to the number of vaccinations. From the public health viewpoint, these findings are critical and no previous study revealed this relation.
Several biologic mechanisms have been proposed to explain the association between kidney function impairment and RCC. In brief, kidney dysfunction results in a state of chronic inflammatory and oxidative stress [39, 40] that provides a microenvironment favouring cancer development [41]. Furthermore, severe CKD creates a relative immunodeficiency status [42] that promotes cancer development. Studies have examined if certain medications for CKD patients (e.g. statins, antihypertensive agents) [43–48] increase cancer risk but found no evidence of their roles in RCC after adjustments for differential longitudinal use of medicines [10]. Several carcinogen-related microorganisms, including human papillomavirus (HPV), hepatitis B and C viruses and Helicobacter pylori, are well known to be associated with cancers. Viral and bacterial infections contracted by CKD patients, such as influenza, pneumonia, pharyngitis and sinusitis, could also create an inflammation and tumourigenic microenvironment [49–51]. Such infections potentially suppress and deteriorate the host immunity, favouring cancer development [51–54]. Cancer is further evolved and confounded by various carcinogen exposures, including smoking and air pollution [55, 56]. In contrast, cancer and precancerous states are related with inhibition of the immune system [49, 52, 57], with these patients at risk of contracting infections. Thus the mutual influence between infections and cancers regarding chronic inflammation and immunity before cancer development is an interesting issue to be investigated.
Infection is an important cause of non-cardiovascular morbidity and mortality among CKD patients [58]. Therefore, the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices and the Kidney Disease: Improving Global Outcomes guidelines recommend annual seasonal influenza vaccines for CKD patients (Grade 1A) [59, 60]. Indeed, influenza vaccination has been shown to reduce the risk for pneumonia-/influenza-related hospitalizations and mortality [61–63]. Our previous study also revealed a reduction in the risk of lung cancer with influenza vaccination among diabetic patients [64]. Another study indicated that the frequency and severity of influenza infections were positively related to the risk of lung cancer events [65], however, the relation between many other solid cancers and influenza events is still unclear.
Aging, smoking and dialysis therapies increase the risk of RCC in CKD patients [66]. From our study, we found a possible relation between influenza vaccination and RCC risk in elderly CKD patients. We also found possible protective effects of influenza vaccination in patients ≥55 years of age in both sexes, which was significant in patients ≥75 years of age [65]. Since the underlying immunologic dysfunction of CKD is related with RCC risk [13] [67], the influenza vaccination–induced immunomodulation might explain its possible protective effects. Dialysis patients had a several-fold increase in the prevalence of RCC, regardless of the underlying cause [68–70]. We found that dialysis patients with influenza vaccination had a lower risk of RCC than those who were unvaccinated. The effects of influenza vaccination on dialysis patients still need to be studied.
Any innate immunity disruption through chemocarcinogen exposure in precancerous states, including chronic inflammation, smoking, environmental pollution or genetic conditions, might predispose to cancers [52, 53, 55]. Our data lack information on such chemocarcinogen exposure such as air pollution and smoking, genetics or lifestyles, and other comorbidities. As an observational study, we also had no available data on other possible precancerous indicators related with oncogenesis, as monitored by immunity-related cells such as T-helper cells and B cells [71–73]. In this precancer period, possible aging and cumulative infection events increased the immune suppression and cancer promotion [49, 74]. This might explain our finding of a potential association between the total number of vaccinations and RCC-free survival years. Moreover, individuals who were vaccinated often had more contact with the healthcare system and might otherwise be in better health (e.g. tobacco, better control of comorbidities etc.).
Our study had several notable strengths. First, the total number of patients analysed in our study represents the largest sample size in published cohort studies examining RCC risk in CKD patients. Our study focused on CKD patients and excluded individuals being transplanted. Second, we decreased potential selection bias by using propensity score matching [35] and adjusted the statistical analyses for potential confounders. Third, the results of subgroup analyses of the protective effects of the influenza vaccination remained consistent.
This study also had several limitations. CKD and RCC diagnoses were ICD-9-CM based, which might be affected by diagnostic accuracy. However, Taiwan has launched nationwide CKD program, and all of ICD coding is uniformly used countrywide. This concern was mitigated by enrolling patients with at least two outpatient clinic records or at least one inpatient clinic record. Taiwan NHIRD data did not provide detailed lifestyle or RCC risk information, such as smoking, environmental exposures, occupation, nutrition, functional status and family history of cancers including RCC.
CONCLUSION
The current study showed that influenza vaccinations associated with a lower risk of RCC in elderly CKD, including dialysis, patients. We recommend annual influenza vaccinations in elderly CKD patients regardless of sex and comorbid conditions, and additional or booster influenza vaccinations might be considered. Whether the RCC preventive effects result from decreased influenza/pneumonia events or come directly from the influenza vaccine remain unclear. Future clinical studies are warranted to examine the causal relationship of the influenza vaccine and RCC in CKD patients, as well as molecular studies to investigate the underlying mechanisms of the vaccine.
Contributor Information
Chia-Wei Lin, Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan; Department of Urology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
Jing-Quan Zheng, Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; Division of Pulmonary Medicine, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan; Division of Pulmonary Medicine, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
Kai-Yi Tzou, Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan; Department of Urology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; Taipei Medical University Research Centre of Urology and Kidney, Taipei Medical University, Taipei, Taiwan.
Yu-Ann Fang, Taipei Heart Institute, Taipei Medical University, Taipei, Taiwan; Division of Cardiology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan.
Wei-Tang Kao, Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan; Department of Urology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; Taipei Medical University Research Centre of Urology and Kidney, Taipei Medical University, Taipei, Taiwan.
Hsin-Ting Lin, Department of Ophthalmology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan; Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan.
Ju-Chi Liu, Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; Taipei Heart Institute, Taipei Medical University, Taipei, Taiwan; Division of Cardiology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan; Division of Cardiology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
Yu-Han Huang, Department of Radiology, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
Yuh-Feng Lin, Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan; Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; Division of Nephrology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan.
Kuo-Cheng Lu, Division of Nephrology, Department of Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan.
Shao-Wei Dong, Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan; Department of Urology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
Cai-Mei Zheng, Taipei Medical University Research Centre of Urology and Kidney, Taipei Medical University, Taipei, Taiwan; Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; Division of Nephrology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan.
Chia-Chang Wu, Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan; Department of Urology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; Taipei Medical University Research Centre of Urology and Kidney, Taipei Medical University, Taipei, Taiwan.
FUNDING
This study was supported by the Taipei Medical University (TMU-IIT1091-15) and Ministry of Science and Technology, Taiwan (MOST 109-2314-B-038-078-MY3, MOST 109-2314-B-038-088, MOST 110-2314-B-038-075-MY3 and MOST 110-2314-B-038-140).
AUTHORS’ CONTRIBUTIONS
Conceptualization, L.-C.W. and W.-C.C.; Methodology, Z.-J.Q. and F.-Y.A.; Software, T.-K.Y. and K.-W.T.; Validation, L.-H.T., L.-J.C. and H.-Y.H.; Formal Analysis, L.-Y.F., L.K.C. and D.-S.W.; Investigation, C.-R.C., C.-Y.C., K.-C.C. and C.-C.P.; Resources, C.-C.P.; Writing—Original Draft Preparation, Z.-C.M., W.C.C., L.-C.W. and Z.-J.Q.; Writing—Review & Editing, L.-C.W.; L.H.T., F.-Y.A. and K.-W.T.; Supervision, Z.-C.M. and W.C.C. Project Administration, L.-K.C.; Funding Acquisition, Z.-C.M. and W.-C.C. All authors have read and agreed to the published version of the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
(See related article by Marques da Silva et al. The potential association between influenza vaccination and lower incidence of renal cell carcinoma. Clin Kidney J (2023) 16: 1714–1717.)
REFERENCES
- 1. Kuo HW, Tsai SS, Tiao MMet al. Epidemiological features of CKD in Taiwan. Am J Kidney Dis 2007;49:46–55. 10.1053/j.ajkd.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 2. Saran R, Li Y, Robinson Bet al. US Renal Data System 2015 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2016;67(3 Suppl 1):Svii, S1–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7:245–57. 10.1038/nrurol.2010.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang WC, Levey AS, Serio AMet al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006;7:735–40. 10.1016/S1470-2045(06)70803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Engels EA, Pfeiffer RM, Fraumeni JF Jret al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891–901. 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maisonneuve P, Agodoa L, Gellert Ret al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet 1999;354:93–9. 10.1016/S0140-6736(99)06154-1 [DOI] [PubMed] [Google Scholar]
- 7. Stewart JH, Buccianti G, Agodoa Let al. Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 2003;14:197–207. 10.1097/01.ASN.0000039608.81046.81 [DOI] [PubMed] [Google Scholar]
- 8. Stewart JH, Vajdic CM, van Leeuwen MTet al. The pattern of excess cancer in dialysis and transplantation. Nephrol Dial Transplant 2009;24:3225–31. 10.1093/ndt/gfp331 [DOI] [PubMed] [Google Scholar]
- 9. Vajdic CM, McDonald SP, McCredie MRet al. Cancer incidence before and after kidney transplantation. JAMA 2006;296:2823–31. 10.1001/jama.296.23.2823 [DOI] [PubMed] [Google Scholar]
- 10. Lowrance WT, Ordonez J, Udaltsova Net al. CKD and the risk of incident cancer. J Am Soc Nephrol 2014;25:2327–34. 10.1681/ASN.2013060604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong G, Hayen A, Chapman JRet al. Association of CKD and cancer risk in older people. J Am Soc Nephrol 2009;20:1341–50. 10.1681/ASN.2008090998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fried LF, Katz R, Sarnak MJet al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 2005;16:3728–35. 10.1681/ASN.2005040384 [DOI] [PubMed] [Google Scholar]
- 13. Kato S, Chmielewski M, Honda Het al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008;3:1526–33. 10.2215/CJN.00950208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenzweig B, Recabal P, Gluck Cet al. Can kidney parenchyma metabolites serve as prognostic biomarkers for long-term kidney function after nephrectomy for renal cell carcinoma? A preliminary study. Clin Kidney J 2021;14:656–64. 10.1093/ckj/sfaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCredie M, Stewart J, Smith Det al. Observations on the effect of abolishing analgesic abuse and reducing smoking on cancers of the kidney and bladder in New South Wales, Australia, 1972–1995. Cancer Causes Control 1999;10:303–11. 10.1023/A:1008900319043 [DOI] [PubMed] [Google Scholar]
- 16. Hunt JD, van der Hel OL, McMillan GPet al. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer 2005;114:101–8. 10.1002/ijc.20618 [DOI] [PubMed] [Google Scholar]
- 17. Colt JS, Schwartz K, Graubard BIet al. Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 2011;22:797–804. 10.1097/EDE.0b013e3182300720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schlehofer B, Pommer W, Mellemgaard Aet al. International renal-cell-cancer study. VI. the role of medical and family history. Int J Cancer 1996;66:723–6. [DOI] [PubMed] [Google Scholar]
- 19. McLaughlin JK, Blot WJ, Mehl ESet al. Petroleum-related employment and renal cell cancer. J Occup Med 1985;27:672–4. [PubMed] [Google Scholar]
- 20. Cho E, Curhan G, Hankinson SEet al. Prospective evaluation of analgesic use and risk of renal cell cancer. Arch Intern Med 2011;171:1487–93. 10.1001/archinternmed.2011.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bowman BT, Rosner MH. Influenza and the patient with end-stage renal disease. J Nephrol 2018;31:225–30. 10.1007/s40620-017-0407-9 [DOI] [PubMed] [Google Scholar]
- 22. Ishigami J, Matsushita K. Clinical epidemiology of infectious disease among patients with chronic kidney disease. Clin Exp Nephrol 2019;23:437–47. 10.1007/s10157-018-1641-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barnes M, Heywood AE, Mahimbo Aet al. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart 2015;101:1738–47. 10.1136/heartjnl-2015-307691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gopal R, Marinelli MA, Alcorn JF. Immune mechanisms in cardiovascular diseases associated with viral infection. Front Immunol 2020;11:570681. 10.3389/fimmu.2020.570681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen CI, Kao PF, Wu MYet al. Influenza vaccination is associated with lower risk of acute coronary syndrome in elderly patients with chronic kidney disease. Medicine (Baltimore) 2016;95:e2588. 10.1097/MD.0000000000002588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dalrymple LS, Go AS. Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol 2008;3:1487–93. 10.2215/CJN.01290308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan ZK, Ikonomidis G, Lazenby Aet al. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med 1995;1:471–7. 10.1038/nm0595-471 [DOI] [PubMed] [Google Scholar]
- 28. Pan ZK, Ikonomidis G, Pardoll Det al. Regression of established tumors in mice mediated by the oral administration of a recombinant Listeria monocytogenes vaccine. Cancer Res 1995;55:4776–9. [PubMed] [Google Scholar]
- 29. Newman JH, Chesson CB, Herzog NLet al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc Natl Acad Sci USA 2020;117:1119–28. 10.1073/pnas.1904022116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan TC, Fu YC, Wang DWet al. Determinants of receiving the pandemic (H1N1) 2009 vaccine and intention to receive the seasonal influenza vaccine in Taiwan. PLoS One 2014;9:e101083. 10.1371/journal.pone.0101083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kao P-F, Liu J-C, Hsu Y-Pet al. Influenza vaccination might reduce the risk of ischemic stroke in patients with atrial fibrillation: a population-based cohort study. Oncotarget 2017;8:112697–711. 10.18632/oncotarget.22352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu JC, Wang TJ, Sung LCet al. Influenza vaccination reduces hemorrhagic stroke risk in patients with atrial fibrillation: a population-based cohort study. Int J Cardiol 2017;232:315–23. 10.1016/j.ijcard.2016.12.074 [DOI] [PubMed] [Google Scholar]
- 33. Fang YA, Chen CI, Liu JCet al. Influenza vaccination reduces hospitalization for heart failure in elderly patients with chronic kidney disease: a population-based cohort study. Acta Cardiol Sin 2016;32:290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Su VY, Yang KY, Yang YHet al. Use of ICS/LABA combinations or LAMA is associated with a lower risk of acute exacerbation in patients with coexistent COPD and asthma. J Allergy Clin Immunol Pract 2018;6:1927–35.e3. 10.1016/j.jaip.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 35. Lévesque LE, Hanley JA, Kezouh Aet al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340:b5087. 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
- 36. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 37. Heinze G, Jüni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J 2011;32:1704–8. 10.1093/eurheartj/ehr031 [DOI] [PubMed] [Google Scholar]
- 38. Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006;15:291–303. 10.1002/pds.1200 [DOI] [PubMed] [Google Scholar]
- 39. Muntner P, Hamm LL, Kusek JWet al. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 2004;140:9–17. 10.7326/0003-4819-140-1-200401060-00006 [DOI] [PubMed] [Google Scholar]
- 40. Shlipak MG, Fried LF, Crump Cet al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 2003;107:87–92. 10.1161/01.CIR.0000042700.48769.59 [DOI] [PubMed] [Google Scholar]
- 41. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giacchino F, Alloatti S, Quarello Fet al. The immunological state in chronic renal insufficiency. Int J Artif Organs 1982;5:237–42. 10.1177/039139888200500406 [DOI] [PubMed] [Google Scholar]
- 43. Bangalore S, Kumar S, Kjeldsen SEet al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol 2011;12:65–82. 10.1016/S1470-2045(10)70260-6 [DOI] [PubMed] [Google Scholar]
- 44. Pahor M, Guralnik JM, Ferrucci Let al. Calcium-channel blockade and incidence of cancer in aged populations. Lancet 1996;348:493–7. 10.1016/S0140-6736(96)04277-8 [DOI] [PubMed] [Google Scholar]
- 45. Andriole GL, Bostwick DG, Brawley OWet al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010;362:1192–202. 10.1056/NEJMoa0908127 [DOI] [PubMed] [Google Scholar]
- 46. Taylor ML, Wells BJ, Smolak MJ. Statins and cancer: a meta-analysis of case-control studies. Eur J Cancer Prev 2008;17:259–68. 10.1097/CEJ.0b013e3282b721fe [DOI] [PubMed] [Google Scholar]
- 47. Chou YC, Lin CH, Wong CSet al. Statin use and the risk of renal cell carcinoma: national cohort study. J Investig Med 2020;68:776–81. 10.1136/jim-2019-001209 [DOI] [PubMed] [Google Scholar]
- 48. Thompson IM, Goodman PJ, Tangen CMet al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–24. 10.1056/NEJMoa030660 [DOI] [PubMed] [Google Scholar]
- 49. Kneale GW, Stewart AM. Pre-cancers and liability to other diseases. Br J Cancer 1978;37:448–57. 10.1038/bjc.1978.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anderson LA, Landgren O, Engels EA. Common community acquired infections and subsequent risk of chronic lymphocytic leukaemia. Br J Haematol 2009;147:444–9. 10.1111/j.1365-2141.2009.07849.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Martel C, Franceschi S. Infections and cancer: established associations and new hypotheses. Crit Rev Oncol Hematol 2009;70:183–94. 10.1016/j.critrevonc.2008.07.021 [DOI] [PubMed] [Google Scholar]
- 52. Hagerling C, Casbon AJ, Werb Z. Balancing the innate immune system in tumor development. Trends Cell Biol 2015;25:214–20. 10.1016/j.tcb.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mantovani A, Allavena P, Sica Aet al. Cancer-related inflammation. Nature 2008;454:436–44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 54. Plummer M, de Martel C, Vignat Jet al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016;4:e609–16. 10.1016/S2214-109X(16)30143-7 [DOI] [PubMed] [Google Scholar]
- 55. Eldridge RC, Pawlita M, Wilson Let al. Smoking and subsequent human papillomavirus infection: a mediation analysis. Ann Epidemiol 2017;27:724–30.e1. 10.1016/j.annepidem.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reuter S, Gupta SC, Chaturvedi MMet al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol Med 2010;49:1603–16. 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tsai HT, Caporaso NE, Kyle RAet al. Evidence of serum immunoglobulin abnormalities up to 9.8 years before diagnosis of chronic lymphocytic leukemia: a prospective study. Blood 2009;114:4928–32. 10.1182/blood-2009-08-237651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saran R, Robinson B, Abbott KCet al. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2019;73:A7–8. 10.1053/j.ajkd.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grohskopf LA, Alyanak E, Ferdinands JMet al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2021–22 influenza season. MMWR Recomm Rep 2021;70:1–28. 10.15585/mmwr.rr7005a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Inker LA, Astor BC, Fox CHet al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–35. 10.1053/j.ajkd.2014.01.416 [DOI] [PubMed] [Google Scholar]
- 61. Remschmidt C, Wichmann O, Harder T. Influenza vaccination in patients with end-stage renal disease: systematic review and assessment of quality of evidence related to vaccine efficacy, effectiveness, and safety. BMC Med 2014;12:244. 10.1186/s12916-014-0244-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang IK, Lin CL, Lin PCet al. Effectiveness of influenza vaccination in patients with end-stage renal disease receiving hemodialysis: a population-based study. PLoS One 2013;8:e58317. 10.1371/journal.pone.0058317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ishigami J, Sang Y, Grams MEet al. effectiveness of influenza vaccination among older adults across kidney function: pooled analysis of 2005–2006 through 2014–2015 influenza seasons. Am J Kidney Dis 2020;75:887–96. 10.1053/j.ajkd.2019.09.008 [DOI] [PubMed] [Google Scholar]
- 64. Zheng JQ, Lin CH, Chen CCet al. Role of annual influenza vaccination against lung cancer in type 2 diabetic patients from a population-based cohort study. J Clin Med 2021;10: 3434. 10.3390/jcm10153434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Weng CF, Chen LJ, Lin CWet al. Association between the risk of lung cancer and influenza: a population-based nested case-control study. Int J Infect Dis 2019;88:8–13. 10.1016/j.ijid.2019.07.030 [DOI] [PubMed] [Google Scholar]
- 66. Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol 2010;23:253–62. [PMC free article] [PubMed] [Google Scholar]
- 67. Vamvakas S, Bahner U, Heidland A. Cancer in end-stage renal disease: potential factors involved -editorial. Am J Nephrol 1998;18:89–95. 10.1159/000013314 [DOI] [PubMed] [Google Scholar]
- 68. Akerlund J, Holmberg E, Lindblad Pet al. Increased risk for renal cell carcinoma in end stage renal disease – a population-based case-control study. Scand J Urol 2021;55:209–14. 10.1080/21681805.2021.1900387 [DOI] [PubMed] [Google Scholar]
- 69. Kojima Y, Takahara S, Miyake Oet al. Renal cell carcinoma in dialysis patients: a single center experience. Int J Urol 2006;13:1045–8. 10.1111/j.1442-2042.2006.01498.x [DOI] [PubMed] [Google Scholar]
- 70. Denton MD, Magee CC, Ovuworie Cet al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int 2002;61:2201–9. 10.1046/j.1523-1755.2002.00374.x [DOI] [PubMed] [Google Scholar]
- 71. Khan AA, Khan Z, Warnakulasuriya S. Cancer-associated toll-like receptor modulation and insinuation in infection susceptibility: association or coincidence? Ann Oncol 2016;27:984–97. 10.1093/annonc/mdw053 [DOI] [PubMed] [Google Scholar]
- 72. Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 2012;22:33–40. 10.1016/j.semcancer.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 73. Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol 2018;59:391–412. 10.1016/j.intimp.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Singh R, Mishra MK, Aggarwal H. Inflammation, immunity, and cancer. Mediators Inflamm 2017;2017:6027305. 10.1155/2017/6027305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.